The plasminogen activator system: involvement in central nervous system inflammation and a potential site for therapeutic intervention
- PMID: 24120085
- PMCID: PMC3852474
- DOI: 10.1186/1742-2094-10-124
The plasminogen activator system: involvement in central nervous system inflammation and a potential site for therapeutic intervention
Abstract
Background: Extracellular proteases such as plasminogen activators (PAs) and matrix metalloproteinases modulate cell-cell and cell-matrix interactions. Components of the PA/plasmin system have been shown to be increased in areas of inflammation, and have been suggested to play a role in inflammatory neurologic disorders such as epilepsy, stroke, brain trauma, Alzheimer's' disease and multiple sclerosis (MS). In the present study, we evaluated the involvement of the PA system in the animal model of MS, experimental autoimmune encephalomyelitis (EAE).
Methods: EAE was induced by myelin oligodendrocyte glycoprotein (MOG) in mice deficient for the urokinase PA (uPA-/-), or the urokinase PA receptor (uPAR-/-). Mice were evaluated for EAE clinical signs and histopathologic parameters, and compared with wild-type (WT) EAE mice. Lymphocytes from the knockout (KO) and WT mice were analyzed for ex vivo restimulation, cytokine secretion, and antigen presentation. Finally, WT EAE mice were treated with PAI-1dp, an 18 amino acid peptide derived from the PA inhibitor protein (PAI-1).
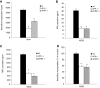
Results: EAE was aggravated in uPA-/- and uPAR-/- mice, and this was accompanied by more severe histopathologic features and microglial activation. By contrast, specific T- cell reactivity towards the encephalitogenic antigen MOG was markedly reduced in the KO animals, as shown by a marked reduction in proliferation and pro-inflammatory cytokine secretion in these mice. Antigen presentation was also reduced in all the KO animals, raising an immunologic paradox. When the mice were treated with PAI-1, a peptide derived from the PA system, a marked and significant improvement in EAE was seen. The clinical improvement was linked to reduced T-cell reactivity, further emphasizing the importance of the PA system in immunomodulation during neuroinflammation.
Conclusions: Cumulatively, our results suggest a role for uPA and uPAR in EAE pathogenesis, as exacerbation of disease was seen in their absence. Furthermore, the successful amelioration of EAE by PAI-1 treatment suggests that the PA system can be considered a potential site for therapeutic intervention in the treatment of neuroimmune diseases.
Figures
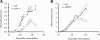



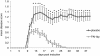
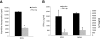
Similar articles
-
Coordinated induction of extracellular proteolysis systems during experimental autoimmune encephalomyelitis in mice.Am J Pathol. 2001 Dec;159(6):2227-37. doi: 10.1016/S0002-9440(10)63073-8. Am J Pathol. 2001. PMID: 11733372 Free PMC article.
-
A role for the plasminogen activator system in inflammation and neurodegeneration in the central nervous system during experimental allergic encephalomyelitis.Am J Pathol. 2005 Aug;167(2):545-54. doi: 10.1016/S0002-9440(10)62996-3. Am J Pathol. 2005. PMID: 16049338 Free PMC article.
-
De novo central nervous system processing of myelin antigen is required for the initiation of experimental autoimmune encephalomyelitis.J Immunol. 2002 Apr 15;168(8):4173-83. doi: 10.4049/jimmunol.168.8.4173. J Immunol. 2002. PMID: 11937578
-
Role of Th17 cells in the pathogenesis of CNS inflammatory demyelination.J Neurol Sci. 2013 Oct 15;333(1-2):76-87. doi: 10.1016/j.jns.2013.03.002. Epub 2013 Apr 8. J Neurol Sci. 2013. PMID: 23578791 Free PMC article. Review.
-
Plasminogen activators and matrix metalloproteases, mediators of extracellular proteolysis in inflammatory demyelination of the central nervous system.J Neuroimmunol. 1999 Feb 1;94(1-2):1-14. doi: 10.1016/s0165-5728(98)00241-0. J Neuroimmunol. 1999. PMID: 10376931 Review.
Cited by
-
Neurodegeneration and inflammation crosstalk: Therapeutic targets and perspectives.IBRO Neurosci Rep. 2022 Dec 16;14:95-110. doi: 10.1016/j.ibneur.2022.12.003. eCollection 2023 Jun. IBRO Neurosci Rep. 2022. PMID: 37388502 Free PMC article.
-
Curbing Inflammation in Multiple Sclerosis and Endometriosis: Should Mast Cells Be Targeted?Int J Inflam. 2015;2015:452095. doi: 10.1155/2015/452095. Epub 2015 Oct 15. Int J Inflam. 2015. PMID: 26550518 Free PMC article. Review.
-
Neurodegeneration and Inflammation-An Interesting Interplay in Parkinson's Disease.Int J Mol Sci. 2020 Nov 10;21(22):8421. doi: 10.3390/ijms21228421. Int J Mol Sci. 2020. PMID: 33182554 Free PMC article. Review.
-
Plasminogen Activator Inhibitor-1 Antagonist TM5484 Attenuates Demyelination and Axonal Degeneration in a Mice Model of Multiple Sclerosis.PLoS One. 2015 Apr 27;10(4):e0124510. doi: 10.1371/journal.pone.0124510. eCollection 2015. PLoS One. 2015. PMID: 25915660 Free PMC article.
-
Proteases in Pemphigoid Diseases.Front Immunol. 2019 Jun 26;10:1454. doi: 10.3389/fimmu.2019.01454. eCollection 2019. Front Immunol. 2019. PMID: 31297118 Free PMC article. Review.
References
-
- Werb Z. ECM and cell surface proteolysis: regulating cellular ecology. Cell. 1997;91:439–442. - PubMed
-
- Matrisian LM. Metalloproteinases and their inhibitors in matrix remodeling. Trends Genet. 1990;6:121–125. - PubMed
-
- Birkedal-Hansen H. Proteolytic remodeling of extracellular matrix. Curr Opin Cell Biol. 1995;7:728–735. - PubMed
-
- Lo EH, Wang X, Cuzner ML. Extracellular proteolysis in brain injury and inflammation: role for plasminogen activators and matrix metalloproteinases. J Neurosci Res. 2002;69:1–9. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

