A single peptide-major histocompatibility complex ligand triggers digital cytokine secretion in CD4(+) T cells
- PMID: 24120362
- PMCID: PMC3846396
- DOI: 10.1016/j.immuni.2013.08.036
A single peptide-major histocompatibility complex ligand triggers digital cytokine secretion in CD4(+) T cells
Abstract
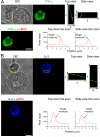
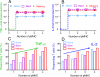
We have developed a single-molecule imaging technique that uses quantum-dot-labeled peptide-major histocompatibility complex (pMHC) ligands to study CD4(+) T cell functional sensitivity. We found that naive T cells, T cell blasts, and memory T cells could all be triggered by a single pMHC to secrete tumor necrosis factor-α (TNF-α) and interleukin-2 (IL-2) cytokines with a rate of ∼1,000, ∼10,000, and ∼10,000 molecules/min, respectively, and that additional pMHCs did not augment secretion, indicating a digital response pattern. We also found that a single pMHC localized to the immunological synapse induced the slow formation of a long-lasting T cell receptor (TCR) cluster, consistent with a serial engagement mechanism. These data show that scaling up CD4(+) T cell cytokine responses involves increasingly efficient T cell recruitment rather than greater cytokine production per cell.
Copyright © 2013 Elsevier Inc. All rights reserved.
Figures
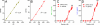
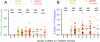


Comment in
-
Digital response in T cells: to be or not to be.Cell Res. 2014 Mar;24(3):265-6. doi: 10.1038/cr.2014.5. Epub 2014 Jan 10. Cell Res. 2014. PMID: 24407423 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

