Protein coated microcrystals formulated with model antigens and modified with calcium phosphate exhibit enhanced phagocytosis and immunogenicity
- PMID: 24120484
- PMCID: PMC4101235
- DOI: 10.1016/j.vaccine.2013.09.061
Protein coated microcrystals formulated with model antigens and modified with calcium phosphate exhibit enhanced phagocytosis and immunogenicity
Abstract
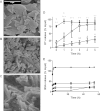
Protein-coated microcrystals (PCMCs) were investigated as potential vaccine formulations for a range of model antigens. Presentation of antigens as PCMCs increased the antigen-specific IgG responses for all antigens tested, compared to soluble antigens. When compared to conventional aluminium-adjuvanted formulations, PCMCs modified with calcium phosphate (CaP) showed enhanced antigen-specific IgG responses and a decreased antigen-specific IgG1:IgG2a ratio, indicating the induction of a more balanced Th1/Th2 response. The rate of antigen release from CaP PCMCs, in vitro, decreased strongly with increasing CaP loading but their immunogenicity in vivo was not significantly different, suggesting the adjuvanticity was not due to a depot effect. Notably, it was found that CaP modification enhanced the phagocytosis of fluorescent antigen-PCMC particles by J774.2 murine monocyte/macrophage cells compared to soluble antigen or soluble PCMCs. Thus, CaP PCMCs may provide an alternative to conventional aluminium-based acellular vaccines to provide a more balanced Th1/Th2 immune response.
Keywords: Adjuvant; Calcium phosphate; Microparticles; Phagocytosis.
Copyright © 2014 The Authors. Published by Elsevier Ltd.. All rights reserved.
Figures


References
-
- Leroux-Roels G. Unmet needs in modern vaccinology: adjuvants to improve the immune response. Vaccine. 2010;28:C25–C36. - PubMed
-
- Gupta R.K. Aluminum compounds as vaccine adjuvants. Adv Drug Deliver Rev. 1998;32:155–172. - PubMed
-
- Schlehuber L.D., McFadyen I.J., Shu Y., Carignan J., Duprex W.P., Forsyth W.R. Towards ambient temperature-stable vaccines: the identification of thermally stabilizing liquid formulations for measles virus using an innovative high-throughput infectivity assay. Vaccine. 2011;29(31):5031–5039. - PubMed
-
- Brandau D.T., Jones L.S., Wiethoff C.M., Rexroad J., Middaugh C.R. Thermal stability of vaccines. J Pharmacol Sci. 2003;92:218–231. - PubMed
-
- Murdan S., Somavarapu S., Ross A.C., Alpar H.O., Parker M.C. Immobilisation of vaccines onto micro-crystals for enhanced thermal stability. Int J Pharm. 2005;296:117–121. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

