Binocular stereoscopy in visual areas V-2, V-3, and V-3A of the macaque monkey
- PMID: 24122139
- PMCID: PMC4074265
- DOI: 10.1093/cercor/bht288
Binocular stereoscopy in visual areas V-2, V-3, and V-3A of the macaque monkey
Abstract
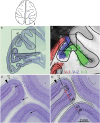
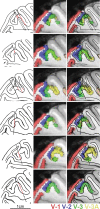
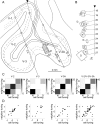
Over 40 years ago, Hubel and Wiesel gave a preliminary report of the first account of cells in monkey cerebral cortex selective for binocular disparity. The cells were located outside of V-1 within a region referred to then as "area 18." A full-length manuscript never followed, because the demarcation of the visual areas within this region had not been fully worked out. Here, we provide a full description of the physiological experiments and identify the locations of the recorded neurons using a contemporary atlas generated by functional magnetic resonance imaging; we also perform an independent analysis of the location of the neurons relative to an anatomical landmark (the base of the lunate sulcus) that is often coincident with the border between V-2 and V-3. Disparity-tuned cells resided not only in V-2, the area now synonymous with area 18, but also in V-3 and probably within V-3A. The recordings showed that the disparity-tuned cells were biased for near disparities, tended to prefer vertical orientations, clustered by disparity preference, and often required stimulation of both eyes to elicit responses, features strongly suggesting a role in stereoscopic depth perception.
Keywords: Hubel and Wiesel, stereopsis; binocular vision; depth; extrastriate cortex; functional organization.
© The Author 2013. Published by Oxford University Press. All rights reserved. For Permissions, please e-mail: journals.permissions@oup.com.
Figures







References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

