Quantitative proton MR techniques for measuring fat
- PMID: 24123229
- PMCID: PMC4001818
- DOI: 10.1002/nbm.3025
Quantitative proton MR techniques for measuring fat
Abstract
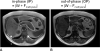
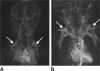
Accurate, precise and reliable techniques for the quantification of body and organ fat distributions are important tools in physiology research. They are critically needed in studies of obesity and diseases involving excess fat accumulation. Proton MR methods address this need by providing an array of relaxometry-based (T1, T2) and chemical shift-based approaches. These techniques can generate informative visualizations of regional and whole-body fat distributions, yield measurements of fat volumes within specific body depots and quantify fat accumulation in abdominal organs and muscles. MR methods are commonly used to investigate the role of fat in nutrition and metabolism, to measure the efficacy of short- and long-term dietary and exercise interventions, to study the implications of fat in organ steatosis and muscular dystrophies and to elucidate pathophysiological mechanisms in the context of obesity and its comorbidities. The purpose of this review is to provide a summary of mainstream MR strategies for fat quantification. The article succinctly describes the principles that differentiate water and fat proton signals, summarizes the advantages and limitations of various techniques and offers a few illustrative examples. The article also highlights recent efforts in the MR of brown adipose tissue and concludes by briefly discussing some future research directions.
Keywords: chemical shift; ectopic fat; fat quantification; muscle; obesity; spectroscopy; water-fat MRI; white and brown adipose tissue.
Copyright © 2013 John Wiley & Sons, Ltd.
Figures









References
-
- McAllister EJ, Dhurandhar NV, Keith SW, Aronne LJ, Barger J, Baskin M, Benca RM, Biggio J, Boggiano MM, Eisenmann JC, Elobeid M, Fontaine KR, Gluckman P, Hanlon EC, Katzmarzyk P, Pietrobelli A, Redden DT, Ruden DM, Wang C, Waterland RA, Wright SM, Allison DB. Ten putative contributors to the obesity epidemic. Crit Rev Food Sci Nutr. 2009;49:868–913. - PMC - PubMed
-
- Smith SR, Lovejoy JC, Greenway F, Ryan D, deJonge L, de la Bretonne J, Volafova J, Bray GA. Contributions of total body fat, abdominal subcutaneous adipose tissue compartments, and visceral adipose tissue to the metabolic complications of obesity. Metabolism. 2001;50:425–435. - PubMed
-
- Eckel RH, Grundy SM, Zimmet PZ. The metabolic syndrome. Lancet. 2005;365:1415–1428. - PubMed
-
- Després JP, Lemieux I, Bergeron J, Pibarot P, Mathieu P, Larose E, Rodés-Cabau J, Bertrand OF, Poirier P. Abdominal obesity and the metabolic syndrome: contribution to global cardiometabolic risk. Arterioscler Thromb Vasc Biol. 2008;28:1039–1049. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

