A study to assess COPD Symptom-based Management and to Optimise treatment Strategy in Japan (COSMOS-J) based on GOLD 2011
- PMID: 24124358
- PMCID: PMC3795054
- DOI: 10.2147/COPD.S48298
A study to assess COPD Symptom-based Management and to Optimise treatment Strategy in Japan (COSMOS-J) based on GOLD 2011
Abstract
Background and objective: The Global initiative for chronic Obstructive Lung Disease (GOLD) Committee has proposed a chronic obstructive pulmonary disease (COPD) assessment framework focused on symptoms and on exacerbation risk. This study will evaluate a symptom and exacerbation risk-based treatment strategy based on GOLD in a real-world setting in Japan. Optimal management of COPD will be determined by assessing symptoms using the COPD Assessment Test (CAT) and by assessing the frequency of exacerbations.
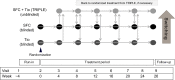
Methods: This study (ClinicalTrials.gov identifier: NCT01762800) is a 24-week, multicenter, randomized, double-blind, double-dummy, parallel-group study. It aims to recruit 400 patients with moderate-to-severe COPD. Patients will be randomized to receive treatment with either salmeterol/fluticasone propionate (SFC) 50/250 μg twice daily or with tiotropium bromide 18 μg once daily. Optimal management of patients will be assessed at four-weekly intervals and, if patients remain symptomatic, as measured using the CAT, or experience an exacerbation, they have the option to step up to treatment with both drugs, ie, SFC twice daily and tiotropium once daily (TRIPLE therapy). The primary endpoint of the study will be the proportion of patients who are able to remain on the randomized therapy.
Results: No data are available. This paper summarizes the methodology of the study in advance of the study starting.
Conclusion: The results of this study will help physicians to understand whether TRIPLE therapy is more effective than either treatment strategy alone in controlling symptoms and exacerbations in patients with moderate-to-severe COPD. It will also help physicians to understand the GOLD recommendation work in Japan.
Keywords: COPD; GOLD; TRIPLE therapy; exacerbation risk; symptom.
Figures
References
-
- The Japanese Respiratory Society . Guidelines for the Diagnosis and Treatment of COPD. 3rd ed. Tokyo: Japanese Respiratory Society; 2009.
-
- Lopez AD, Shibuya K, Rao C, et al. Chronic obstructive pulmonary disease: current burden and future projections. Eur Respir J. 2006;27:397–412. - PubMed
-
- Global initiative for chronic Obstructive Lung Disease (GOLD) [homepage on the Internet] 2010Available from: http://www.goldcopd.org/Guidelines/guideline-2010-gold-report.htmlAccessed March 25, 2013
-
- Global initiative for chronic Obstructive Lung Disease (GOLD) 2011http://www.goldcopd.org/Guidelines/guideline-2011-gold-report.htmlAccessed March 25, 2013
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous