In situ gelation for cell immobilization and culture in alginate foam scaffolds
- PMID: 24125496
- PMCID: PMC3926177
- DOI: 10.1089/ten.TEA.2013.0223
In situ gelation for cell immobilization and culture in alginate foam scaffolds
Abstract
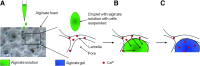
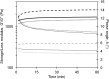
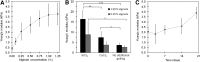
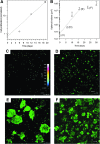
Essential cellular functions are often lost under culture in traditional two-dimensional (2D) systems. Therefore, biologically more realistic three-dimensional (3D) cell culture systems are needed that provide mechanical and biochemical cues which may otherwise be unavailable in 2D. For the present study, an alginate-based hydrogel system was used in which cells in an alginate solution were seeded onto dried alginate foams. A uniform distribution of NIH:3T3 and NHIK 3025 cells entrapped within the foam was achieved by in situ gelation induced by calcium ions integrated in the foam. The seeding efficiency of the cells was about 100% for cells added in a seeding solution containing 0.1-1.0% alginate compared with 18% when seeded without alginate. The NHIK 3025 cells were allowed to proliferate and form multi-cellular structures inside the transparent gel that were later vital stained and evaluated by confocal microscopy. Gels were de-gelled at different time points to isolate the multi-cellular structures and to determine the spheroid growth rate. It was also demonstrated that the mechanical properties of the gel could largely be varied through selection of type and concentration of the applied alginate and by immersing the already gelled disks in solutions providing additional gel-forming ions. Cells can efficiently be incorporated into the gel, and single cells and multi-cellular structures that may be formed inside can be retrieved without influencing cell viability or contaminating the sample with enzymes. The data show that the current system may overcome some limitations of current 3D scaffolds such as cell retrieval and in situ cell staining and imaging.
Figures

References
-
- Pampaloni F., Reynaud E.G., and Stelzer E.H.The third dimension bridges the gap between cell culture and live tissue. Nat Rev Mol Cell Biol 8,839, 2007 - PubMed
-
- Lee J., Cuddihy M.J., and Kotov N.A.Three-dimensional cell culture matrices: state of the art. Tissue Eng Part B Rev 14,61, 2008 - PubMed
-
- Justice B.A., Badr N.A., and Felder R.A.3D cell culture opens new dimensions in cell-based assays. Drug Discov Today 14,102, 2009 - PubMed
-
- Reilly G.C., and Engler A.J.Intrinsic extracellular matrix properties regulate stem cell differentiation. J Biomech 43,55, 2010 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

