Long-acting beta2-agonists for chronic obstructive pulmonary disease
- PMID: 24127118
- PMCID: PMC11663505
- DOI: 10.1002/14651858.CD010177.pub2
Long-acting beta2-agonists for chronic obstructive pulmonary disease
Abstract
Background: Chronic obstructive pulmonary disease (COPD) is a respiratory disease that causes progressive symptoms of breathlessness, cough and mucus build-up. It is the fourth or fifth most common cause of death worldwide and is associated with significant healthcare costs.Inhaled long-acting beta2-agonists (LABAs) are widely prescribed to manage the symptoms of COPD when short-acting agents alone are no longer sufficient. Twice-daily treatment with an inhaled LABA is aimed at relieving symptoms, improving exercise tolerance and quality of life, slowing decline and even improving lung function and preventing and treating exacerbations.
Objectives: To assess the effects of twice-daily long-acting beta2-agonists compared with placebo for patients with COPD on the basis of clinically important endpoints, primarily quality of life and COPD exacerbations.
Search methods: We searched the Cochrane Airways Group trials register, ClinicalTrials.gov and manufacturers' websites in June 2013.
Selection criteria: Parallel, randomised controlled trials (RCTs) recruiting populations of patients with chronic obstructive pulmonary disease. Studies were required to be at least 12 weeks in duration and designed to assess the safety and efficacy of a long-acting beta2-agonist against placebo.
Data collection and analysis: Data and characteristics were extracted independently by two review authors, and each study was assessed for potential sources of bias. Data for all outcomes were pooled and subgrouped by LABA agent (formoterol 12 μg, formoterol 24 μg and salmeterol 50 μg) and then were separately analysed by LABA agent and subgrouped by trial duration. Sensitivity analyses were conducted for the proportion of participants taking inhaled corticosteroids and for studies with high or uneven rates of attrition.
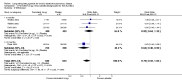
Main results: Twenty-six RCTs met the inclusion criteria, randomly assigning 14,939 people with COPD to receive twice-daily LABA or placebo. Study duration ranged from three months to three years; the median duration was six months. Participants were more often male with moderate to severe symptoms at randomisation; mean forced expiratory volume in 1 second (FEV1) was between 33% and 55% predicted normal in the studies, and mean St George's Respiratory Questionnaire score (SGRQ) ranged from 44 to 55 when reported.Moderate-quality evidence showed that LABA treatment improved quality of life on the SGRQ (mean difference (MD) -2.32, 95% confidence interval (CI) -3.09 to -1.54; I(2) = 50%; 17 trials including 11,397 people) and reduced the number of exacerbations requiring hospitalisation (odds ratio (OR) 0.73, 95% CI 0.56 to 0.95; I(2) = 10%; seven trials including 3804 people). In absolute terms, 18 fewer people per 1000 were hospitalised as the result of an exacerbation while receiving LABA therapy over a weighted mean of 7 months (95% CI 3 to 31 fewer). Scores were also improved on the Chronic Respiratory Disease Questionnaire (CRQ), and more people receiving LABA treatment showed clinically important improvement of at least four points on the SGRQ.The number of people who had exacerbations requiring a course of oral steroids or antibiotics was also lower among those taking LABA (52 fewer per 1000 treated over 8 months; 95% CI 24 to 78 fewer, moderate quality evidence).Mortality was low, and combined findings of all studies showed that LABA therapy did not significantly affect mortality (OR 0.90, 95% CI 0.75 to 1.08; I(2) = 21%; 23 trials including 14,079 people, moderate quality evidence). LABA therapy did not affect the rate of serious adverse events (OR 0.97, 95% CI 0.83 to 1.14; I(2) = 34%, moderate quality evidence), although there was significant unexplained heterogeneity, especially between the two formoterol doses.LABA therapy improved predose FEV1 by 73 mL more than placebo (95% CI 48 to 98; I(2) = 71%, low quality evidence), and people were more likely to withdraw from placebo than from LABA therapy (OR 0.74, 95% CI 0.69 to 0.80; I(2) = 0%). Higher rates of withdrawal in the placebo arm may reduce our confidence in some results, but the disparity is more likely to reduce the magnitude of difference between LABA and placebo than inflate the true effect; removing studies at highest risk of bias on the basis of high and unbalanced attrition did not change conclusions for the primary outcomes.
Authors' conclusions: Moderate-quality evidence from 26 studies showed that inhaled long-acting beta2-agonists are effective over the medium and long term for patients with moderate to severe COPD. Their use is associated with improved quality of life and reduced exacerbations, including those requiring hospitalisation. Overall, findings showed that inhaled LABAs did not significantly reduce mortality or serious adverse events.
Conflict of interest statement
None known.
Figures
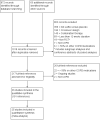











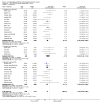


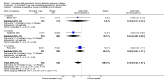


















Update of
References
References to studies included in this review
Aalbers 2002 {published data only}
-
- Aalbers R, Ayres J, Backer V, Decramer M, Lier PA, Magyar P, et al. Formoterol in patients with chronic obstructive pulmonary disease: a randomized, controlled, 3‐month trial. European Respiratory Journal 2002;19(5):936‐43. [0903‐1936] - PubMed
-
- Sybrecht GW. Inhaled formoterol was an effective and safe treatment in COPD patients. European Respiratory Society; 1999 October 9‐13; Madrid, p 2510.
Bogdan 2011 {published data only}
-
- Bogdan MA, Kudo T, Umemiya M. Efficacy and safety of inhaled formoterol 4.5 and 9 1/4g twice daily in Japanese and European patients with COPD: results of a Phase III study [Abstract]. American Journal of Respiratory and Critical Care Medicine 2010;181:A4494.
-
- Ichinose M, Aizawa H, Fukuchi Y, Mishima M, Nishimura M, Bogdan M. Patient‐reported outcomes (PROs) and reliever use in Japanese and European patients with chronic obstructive pulmonary disease receiving formoterol 4.5 and 9 microg twice daily: Results of the OCEAN phase III study [Abstract]. European Respiratory Society Annual Congress; 2010 Sep 18‐22; Barcelona, p 4591.
Brusasco 2003 {published data only}
-
- Bateman ED, Hodder R, Miravitlles M, Lee A, Towse L, Serby C. A comparative trial of tiotropium, salmeterol and placebo: health‐related quality of life. European Respiratory Journal 2001;18(Suppl 33):26s.
-
- Bateman ED, Jenkins C, Korducki L, Keston S. Tiotropium (TIO) improves health care resource utilization (HRU) and patient disability in patients with COPD. American Journal of Respiratory and Critical Care Medicine 2002;165(Suppl 8):A111.
-
- Brusasco V, Menjoge SS, Kesten S. Flow and volume responders following treatment with tiotropium and salmeterol in patients with COPD [abstract]. American Thoracic Society 99th International Conference; 2003 May 16‐21; Seattle, Poster 420.
-
- Brusasco V, Menjoge SS, Kesten S. Flow and volume responders over 12 hours following treatment with tiotropium or salmeterol in patients with COPD [Abstract]. Proceedings of the American Thoracic Society; 2006 May 19‐24; San Diego, A110 [Poster J10].
Calverley 2003a {published data only}
-
- AstraZeneca. A placebo‐controlled 12‐month efficacy study of the fixed combination budesonide/formoterol compared to budesonide and formoterol as monotherapies in patients with Chronic Obstructive Pulmonary Disease (COPD). SD‐039‐0670. http://www.astrazenecaclinicaltrials.com/ (accessed 12 December 2012).
-
- Borgstrom L, Asking L, Olsson H, Peterson S. Lack of interaction between disease severity and therapeutic response with budesonide/formoterol in a single inhaler [Abstract]. American Thoracic Society 100th International Conference; 2004 May 21‐26; Orlando, C22 Poster 505.
-
- Calverley PM, Boonsawat W, Cseke Z, Zhong N, Peterson S, Olsson H. Maintenance therapy with budesonide and formoterol in chronic obstructive pulmonary disease. European Respiratory Journal 2003;22(6):912‐9. [0903‐1936] - PubMed
-
- Calverley PM, Szafranski W, Anderson JA. Budesonide/formoterol is a well‐tolerated long term maintenance therapy for COPD [Abstract]. European Respiratory Journal 2005;26(Suppl 49):Abstract No. 1917.
-
- Calverley PMA, Cseke Z, Peterson S. Budesonide/formoterol reduces the use of oral corticosteroids in the treatment of COPD [Abstract]. European Respiratory Journal 2003;22(Suppl 45):Abstract No. P436.
Calverley 2003b [TRISTAN] {published data only}
-
- Calverley P, Pauwels R, Vestbo J. Erratum: Combined salmeterol and fluticasone in the treatment of chronic obstructive pulmonary disease: a randomised controlled trial. Lancet 2003;361(9369):449‐56. [0140‐6736] - PubMed
-
- Calverley P, Pauwels R, Vestbo J, Jones P, Pride N, Gulsvik A, et al. Combined salmeterol and fluticasone in the treatment of chronic obstructive pulmonary disease: a randomised controlled trial. Lancet 2003;361(9356):449‐56. [0140‐6736] - PubMed
-
- Calverley PMA, Pauwels R, Vestbo J, Jones P, Pride NB, Gulsvik A, et al. Safety of salmeterol/fluticasone propionate combination in the treatment of chronic obstructive pulmonary disease (COPD) for one year [abstract]. European Respiratory Society Annual Congress; 2002 Sep 14‐18; Stockholm, Abstract P1572.
-
- Calverley PMA, Pauwels RA, Vestbo J, Jones PW, Pride NB, Gulsvik A, et al. Salmeterol/fluticasone propionate combination for one year provides greater clinical benefit than its individual components in COPD. American Journal of Respiratory and Critical Care Medicine 2002;165(Suppl 8):A226.
Calverley 2007 [TORCH] {published data only}
-
- Allegra L. TORCH study: an invitation to clinical considerations. Giornale Italiano delle Malattie del Torace 2007;61(1):15‐23.
-
- Briggs AH, Glick HA, Lozano‐Ortega G, Spencer M, Calverley PMA, Jones PW, et al. Is treatment with ICS and LABA cost‐effective for COPD? Multinational economic analysis of the TORCH study. European Respiratory Journal 2010;35(3):532‐9. - PubMed
-
- Calverley P, Celli B, Anderson J, Ferguson G, Jenkins C, Jones P, et al. Salmeterol/fluticasone propionate combination (SFC) improves survival in COPD over three years: on‐treatment analysis from the TORCH study [Abstract]. American Thoracic Society International Conference; 2009 May 15‐20; San Diego, A6191 (Poster 219).
-
- Calverley P, Celli B, Anderson JA, Ferguson GT, Jenkins C, Jones PW, et al. The TOwards a Revolution in COPD Health (TORCH) study: salmeterol/fluticasone propionate improves survival in COPD over three years [Abstract]. Respirology 2006;11(Suppl 5):A149 [PS‐3‐8].
-
- Calverley PM, Anderson JA, Celli B, Ferguson GT, Jenkins C, Jones PW, et al. Cardiovascular events in patients with COPD: TORCH study results. Thorax 2010;65(8):719‐25. - PubMed
Campbell 2005 {published data only}
-
- AstraZeneca. A 6‐month study to compare the efficacy and safety of 9microg formoterol (Oxis) Turbuhaler b.i.d. with placebo b.i.d. in patients with Chronic Obstructive Pulmonary Disease (COPD). Furthermore to compare formoterol 4.5microg as needed with terbutaline 0.5 mg as needed. SD‐037‐0709. http://www.astrazenecaclinicaltrials.com/ (accessed 12 December 2012).
-
- Bogdan M, Eliraz A, McKinnon C, Nihlen U, Radeczky E, Soliman S, et al. Formoterol turbuhaler is an effective maintenance and maintenance plus reliever therapy in patients with chronic obstructive pulmonary disease (COPD) irrespective of the level of lung function impairment and reversibility [abstract]. European Respiratory Society Annual Congress. 2002 Sep 14‐18:P1578.
-
- Campbell M, Eliraz A, Johansson G, Tornling G, Nihlen U, Bengtsson T, et al. Formoterol for maintenance and as‐needed treatment of chronic obstructive pulmonary disease. Respiratory Medicine 2005;99(12):1511‐20. - PubMed
-
- Campbell M, Rabe KF, Andersson A. Formoterol turbuhaler for maintenance and as‐needed use is effective and well tolerated in patients with COPD [abstract]. American Thoracic Society 99th International Conference; 2003 May 16‐21; Seattle, B024 Poster 417.
-
- Eliraz A, Bengtsson T, Bogdan M, Coenen PDM, Johanson G, Osmanilev D, et al. Formoterol turbuhaler is effective and safe as maintenance or maintenance plus reliever therapy in patients with chronic obstructive pulmonary disease (COPD) [abstract]. European Respiratory Society Annual Congress; 2002 Sep 14‐18; Stockholm, Abstract P1580.
Dahl 2001 {published data only}
-
- Dahl R, Greefhorst AP, Byrne AM. Onset of inhaled formoterol compared to ipratropium bromide in patients with COPD. European Respiratory Journal 2000;16(Suppl 31):5s.
-
- Dahl R, Greefhorst APM, Nowak D, Nonikov V, Byrne A, Colacchio C, et al. Comparison of the efficacy and safety of inhaled formoterol and ipratropium bromide in patients with COPD. American Journal of Respiratory and Critical Care Medicine 2000;161(Suppl 3):A489.
-
- Dahl R, Greefhorst APM, Thomson MH, Till D. Formoterol (Foradil®) improves lung function and quality of life (QOL) parameters in patients with reversible or poorly reversible COPD. American Journal of Respiratory and Critical Care Medicine 2001;163(Suppl 5):A280.
-
- Dahl R, Greefhorst LA, Nowak D, Nonikov V, Byrne AM, Thomson MH, et al. Inhaled formoterol dry powder versus ipratropium bromide in chronic obstructive pulmonary disease. American Journal of Respiratory and Critical Care Medicine. 2001;164(5):778‐84. [1073‐449X] - PubMed
-
- Dahl R, Kristufek P, Greefhorst APM, Amgott TR, Della Cioppa G, Thompson MH. The cardiac safety profile of formoterol dry powder is similar to placebo in patients with COPD. European Respiratory Journal 2000;16(Suppl 31):51s.
Dahl 2010 {published data only}
-
- Dahl R, Chung KF, Buhl R, Magnussen H, Nonikov V, Jack D, et al. Efficacy of a new once‐daily long‐acting inhaled beta2‐agonist indacaterol versus twice‐daily formoterol in COPD. Thorax 2010;65(6):473‐9. - PubMed
-
- Dahl R, Kolman P, Jack D, Bleasdale P, Owen R, Higgins M, et al. Bronchodilator therapy with indacaterol once‐daily in COPD: a 52‐week comparison with formoterol [Abstract]. European Respiratory Society Annual Congress; 2009 Sep 12‐16; Vienna, E4350.
-
- Jones PW, Mahler DA, Gale R, Owen R, Kramer B. Profiling the effects of indacaterol on dyspnoea and health status in patients with COPD. Respiratory Medicine 2011;105(6):892‐9. - PubMed
-
- Magnussen H, Paggiaro P, Jack D, Owen R, Higgins M, Kramer B, et al. Bronchodilator treatment with indacaterol once‐daily vs formoterol twice‐daily in COPD: a 52‐week study [Abstract]. American Thoracic Society International Conference; 2009 May 15‐20; San Diego, A6184 Poster 211.
-
- Nonikov V, Verkindre C, Jack D, Bleasdale P, Owen R, Kramer B, et al. Indacaterol once‐daily improves symptom control in COPD patients: a 52‐week evaluation vs placebo (pbo) and formoterol (for) [Abstract]. European Respiratory Society Annual Congress; 2009 Sep 12‐16; Vienna, P2024.
Doherty 2012 {published data only}
-
- Doherty D, Tashkin D, Kerwin E, Eduardo Matiz‐Bueno C, Shekar T, Banerjee S, et al. Efficacy and safety of mometasone furoate/formoterol in subjects with moderate to very severe chronic obstructive pulmonary disease: results from two phase three 26‐week trials [Abstract]. Chest 2011;140(4):535A.
-
- Doherty DE, Kerwin E, Tashkin DP, Matiz‐Bueno CE, Shekar T, Banerjee S, et al. Combined mometasone furoate and formoterol in patients with moderate to very severe chronic obstructive pulmonary disease (COPD): phase 3 efficacy and safety study [Abstract]. Journal of Allergy and Clinical Immunology 2012;129(Suppl 2):AB75 [283]. [0091‐6749]
-
- Doherty DE, Tashkin DP, Kerwin E, Knorr BA, Shekar T, Banerjee S, et al. Effects of mometasone furoate/formoterol fumarate fixed‐dose combination formulation on chronic obstructive pulmonary disease (COPD): results from a 52‐week phase III trial in subjects with moderate‐to‐very severe COPD. International Journal of COPD 2012;7:57‐71. - PMC - PubMed
-
- Kerwin E, Tashkin D, Matiz‐Bueno CE, Doherty D, Shekar T, Banerjee S, et al. Quality of life following 26 weeks of mometasone furoate/formoterol therapy: results from two phase three trials in subjects with moderate to very severe chronic obstructive pulmonary disease [Abstract]. Chest 2011;140(4):558A.
-
- Matiz‐Bueno CE, Doherty D, Kerwin E, Tashkin D, Shekar T, Banerjee S, et al. The long‐term safety characteristics of mometasone furoate/formoterol for the treatment of moderate to very severe chronic obstructive pulmonary disease: pooled findings from two 1‐year multicenter clinical trials [Abstract]. Chest 2011;140(4):548A.
Hanania 2003 {published data only}
-
- Ferguson G, Funck‐Brentano C, Fischer T, Darken P, Troy S, Compton C, et al. Cardiovascular safety of salmeterol in patients with COPD. American Journal of Respiratory and Critical Care Medicine 2002;165(Suppl 8):A228.
-
- Ferguson GT, Funck‐Brentano C, Fischer T, Darken P, Reisner C. Cardiovascular safety of salmeterol in COPD. Chest 2003;123(6):1817‐24. - PubMed
-
- GlaxoSmithKline. A randomized, double‐blind, placebo‐controlled, parallel‐group trial evaluating the safety and efficacy of the DISKUS formulations of salmeterol (SAL) 50mcg BID and fluticasone propionate (FP) 250mcg BID individually and in combination as salmeterol 50mcg/fluticasone propionate 250mcg BID (SFC 50/250) compared to placebo in COPD subjects. SFCA3007. http://www.gsk‐clinicalstudyregister.com/ (accessed 14 December 2012).
-
- Hanania NA, Darken P, Horstman D, Reisner C, Lee B, Davis S, et al. The efficacy and safety of fluticasone propionate (250 microg)/salmeterol (50 microg) combined in the Diskus inhaler for the treatment of COPD. Chest 2003;124(3):834‐43. - PubMed
-
- Hanania NA, Knobil K, Watkins M, Wire P, Yates J, Darken P. Salmeterol and fluticasone propionate therapy administered by a single diskus in patients with COPD. [Abstract]. Chest; 2002 Nov 2‐7; San Diego, S129.
Hanrahan 2008 {published data only}
-
- Baumgartner RA, Hanania NA, Calhoun WJ, Sahn SA, Sciarappa K, Hanrahan JP. Nebulized arformoterol in patients with COPD: a 12‐week, multicenter, randomized, double‐blind, double‐dummy, placebo‐ and active‐controlled trial. Clinical Therapeutics 2007;29(2):261‐78. - PubMed
-
- Grogan DR, Hanrahan JP, Morganroth J, Cheng H, Baumgartner RA. Arrythmias in COPD: Holter monitoring results from 2 arformoterol pivotal trials [Abstract]. American Thoracic Society International Conference; 2007 May 18‐23; San Francisco, Poster 413.
-
- Hanrahan JP, Grogan DR, Baumgartner RA, Wilson A, Cheng H, Zimetbaum PJ, et al. Arrhythmias in patients with chronic obstructive pulmonary disease (COPD): occurrence frequency and the effect of treatment with the inhaled long‐acting beta2‐agonists arformoterol and salmeterol. Medicine 2008;87(6):319‐28. - PubMed
-
- Hanrahan JP, Hanania NA, Calhoun WJ, Sahn SA, Sciarappa K, Baumgartner RA. Effect of nebulized arformoterol on airway function in COPD: results from two randomized trials. Journal of Chronic Obstructive Pulmonary Disease 2008;5(1):25‐34. - PubMed
-
- Hanrahan JP, Kerwin E, Cheng H, Grogan DR, Baumgartner RA. Arformoterol in COPD safety results from two pooled phase 3 trials [Abstract]. European Respiratory Journal 2006;28(Suppl 50):428s [P2500].
Kornmann 2011 {published data only}
-
- Kornmann O, Dahl R, Centanni S, Dogra A, Owen R, Lassen C, et al. Once‐daily indacaterol versus twice‐daily salmeterol for COPD: a placebo‐controlled comparison. European Respiratory Journal 2011;37(2):273‐9. - PubMed
-
- Kornmann O, Luthra A, Owen R, Lassen C, Kramer B. Once‐daily indacaterol provides superior bronchodilation, health status and clinical outcomes compared with salmeterol in patients with chronic obstructive COPD: a 26‐week placebo‐controlled study. Chest 2009;136(4):152S.
Mahler 1999 {published data only}
-
- Ferguson G, Funck‐Brentano C, Fischer T, Darken P, Troy S, Compton C, et al. Cardiovascular safety of salmeterol in patients with COPD. American Journal of Respiratory and Critical Care Medicine 2002;165(Suppl 8):A228.
-
- Ferguson GT, Funck‐Brentano C, Fischer T, Darken P, Reisner C. Cardiovascular safety of salmeterol in COPD. Chest 2003;123(6):1817‐24. - PubMed
-
- GlaxoSmithKline. A randomized, double‐blind, double‐dummy, comparative clinical trial of 12‐week courses of salmeterol xinafoate versus ipratropium bromide versus placebo (PRN Ventolin) in subjects with chronic obstructive pulmonary disease. SLGA4005. www.gsk‐clinicalstudyregister.com (accessed 20 December 2012).
-
- Mahler DA, Donohue JF, Barbee RA, Goldman MD, Gross NJ, Wisniewski ME, et al. Efficacy of salmeterol xinafoate in the treatment of COPD. Chest 1999;115(4):957‐65. - PubMed
Mahler 2002 {published data only}
-
- Ferguson G, Funck‐Brentano C, Fischer T, Darken P, Troy S, Compton C, et al. Cardiovascular safety of salmeterol in patients with COPD. American Journal of Respiratory and Critical Care Medicine 2002;165(Suppl 8):A228.
-
- Ferguson GT, Funck‐Brentano C, Fischer T, Darken P, Reisner C. Cardiovascular safety of salmeterol in COPD. Chest 2003;123(6):1817‐24. - PubMed
-
- GlaxoSmithKline. A randomized, double‐blind, placebo‐controlled, parallel‐group trial evaluating the safety and efficacy of the DISKUS formulations of salmeterol (SAL) 50mcg BID and fluticasone propionate (FP) 500mcg BID individually and in combination as salmeterol 50mcg/fluticasone propionate 500mcg BID (SFC 50/500) compared to placebo in COPD subjects. SFCA3006. www.gsk‐clinicalstudyregister.com (accessed 10 December 2012).
-
- Littner M, Yates J, Fischer T, Horstman D, Wire P. Improvements in FEV1 and symptoms in poorly‐reversible COPD patients following 24 weeks of treatment with the salmeterol/fluticasone propionate combination 50/500 BID via the Diskus™ inhaler. European Respiratory Journal 2001;18(Suppl 33):176s.
-
- Mahler DA, Wire P, Horstman D, Chang CN, Yates J, Fischer T, et al. Effectiveness of fluticasone propionate and salmeterol combination delivered via the Diskus device in the treatment of chronic obstructive pulmonary disease. American Journal of Respiratory and Critical Care Medicine 2002;166(8):1084‐91. [1073‐449X] - PubMed
Nelson 2007 {published data only}
-
- Gross NJ, Lapidus R, Dunn LJ, Lynn LD, Denis‐Mize K, Rinehart M. Nebulized formoterol is an effective bronchodilator and improves the quality of life for COPD patients [Abstract]. American Thoracic Society International Conference; 2007 May 18‐23; San Francisco, Poster A10.
-
- Gross NJ, Nelson HS, Lapidus RJ, Dunn L, Lynn L, Rinehart M, et al. Efficacy and safety of formoterol fumarate delivered by nebulization to COPD patients. Respiratory Medicine 2008;102(2):189‐97. - PubMed
-
- Nelson HS, Gross NJ, Levine B, Kerwin EM, Rinehart M, Denis‐Mize K, et al. Cardiac safety profile of nebulized formoterol in adults with COPD: a 12‐week, multicenter, randomized, double‐blind, double‐dummy, placebo‐ and active‐controlled trial. Clinical Therapeutics 2007;29(10):2167‐78. - PubMed
-
- Nelson HS, Gross NJ, Rinehart M, Denis‐Mize K. Cardiovascular safety of nebulized formoterol in COPD patients: a double‐blind, placebo‐controlled study. Chest 2007;132(4):529b‐530.
-
- Nelson HS, ZuWallack R, Levine B, Kerwin EM, Denis‐Mize K, Rinehart M. Safety profile of formoterol fumarate delivered by nebulization to COPD patients [Abstract]. American Thoracic Society International Conference; 2007 May 18‐23; San Francisco, Poster A11.
Rennard 2001 {published data only}
-
- Ferguson G, Funck‐Brentano C, Fischer T, Darken P, Troy S, Compton C, et al. Cardiovascular safety of salmeterol in patients with COPD. American Journal of Respiratory and Critical Care Medicine 2002;165(Suppl 8):A228.
-
- Ferguson GT, Funck‐Brentano C, Fischer T, Darken P, Reisner C. Cardiovascular safety of salmeterol in COPD. Chest 2003;123(6):1817‐24. - PubMed
-
- GlaxoSmithKline. A randomized, double‐blind, double‐dummy, comparative clinical trial of 12‐week courses of salmeterol xinafoate versus ipratropium bromide versus placebo (PRN Ventolin®) in subjects with chronic obstructive pulmonary disease. SLGA4004. www.gsk‐clinicalstudyregister.com (accessed 7 December 2012).
-
- Rennard SI, Anderson W, ZuWallack R, Broughton J, Bailey W, Friedman M, et al. Use of a long‐acting inhaled beta2‐adrenergic agonist, salmeterol xinafoate, in patients with chronic obstructive pulmonary disease. American Journal of Respiratory and Critical Care Medicine 2001;163(5):1087‐92. - PubMed
Rennard 2009 {published data only}
-
- Celli BR, Tashkin DP, Rennard SI, McElhattan J, Martin UJ. Bronchodilator responsiveness and onset of effect with budesonide/formoterol pMDI in COPD. Respiratory Medicine 2011;105(8):1176‐88. - PubMed
-
- Laties A, Rennard SI, Tashkin DP, Suchower LJ, Martin UJ. Effect of budesonide/formoterol pressurized metered‐dose inhaler (bud/fm pMDI) on ophthalmologic assessments in moderate to very severe chronic obstructive pulmonary disease (COPD) patients: results from a 1‐year, randomized, controlled clinical trial [Abstract]. Chest 2010;138(4):468A.
-
- Nelson HS, Tashkin DP, Rennard SI, Martin P, Goldman M, Silkoff PE. Onset of bronchodilation with budesonide and formoterol administered in one pressurized metered‐dose inhaler in patients with moderate to very severe chronic obstructive pulmonary disease [Abstract]. Chest 2008;134(4):105002s. - PubMed
-
- Rennard SI, Tashkin DP, McElhattan J, Goldman M, Ramachandran S, Martin UJ, et al. Efficacy and tolerability of budesonide/formoterol in one hydrofluoroalkane pressurized metered‐dose inhaler in patients with chronic obstructive pulmonary disease: results from a 1‐year randomized controlled clinical trial. Drugs 2009;69(5):549‐65. [0012‐6667] - PMC - PubMed
Rossi 2002 {published data only}
-
- Amgott TR, Kristufek P, Levine B, Byrne A, Till D. Effect of inhaled formoterol and oral slow‐release theophylline on peak expiratory flow and symptoms in patients with COPD. American Journal of Respiratory and Critical Care Medicine 2000;161(Suppl 3):A582.
-
- Kristufek P, Amgott TR, Levine B, Byrne A, Colacchio C. Comparison of the efficacy and safety of inhaled formoterol dry powder and oral slow‐release theophylline in patients with COPD. American Journal of Respiratory and Critical Care Medicine 2000;161(Suppl 3):A489.
-
- Kristufek P, Levine B, Della Cioppa G, Byrne A, Till D. Bronchodilatory effects of formoterol in patients with chronic obstructive pulmonary disease (COPD) are not influenced by concomitant corticosteroid use. European Respiratory Journal 2001;18(Suppl 33):514s.
-
- Kristufek P, Levine B, Till D, Byrne A. Inhaled formoterol (Foradil®) improves lung function in patients with both reversible and poorly reversible COPD. American Journal of Respiratory and Critical Care Medicine 2001;163(Suppl 5):A280.
-
- Rossi A, Kristufek P, Levine BE, Thomson MH, Till D, Kottakis J, et al. Comparison of the efficacy, tolerability, and safety of formoterol dry powder and oral, slow‐release theophylline in the treatment of COPD. Chest 2002;121(4):1058‐69. [0012‐3692] - PubMed
SLMF4010 2005 {published data only}
-
- GlaxoSmithKline. Multicentre, randomised, parallel group, placebo‐controlled, double‐blind study, stratified on tobacco status at enrolment, evaluating during 6 months the efficacy of salmeterol powder for inhalation, 50µg two times per day for the reduction of thoracic distension in subjects with chronic obstructive pulmonary disease (COPD). SLMF4010. www.gsk‐clinicalstudyregister.com (accessed 9 December 2012).
Szafranski 2003 {published data only}
-
- Anderson P. Budesonide/formoterol in a single inhaler (Symbicort®) provides early and sustained improvement in lung function in moderate to severe COPD. Thorax 2002;57(Suppl 3):iii43.
-
- AstraZeneca SD. A placebo‐controlled 12 month efficacy study of the fixed combination budesonide/formoterol compared with budesonide and formoterol as monotherapies in patients with chronic obstructive pulmonary disease (COPD). SD‐039‐CR‐0629. http://www.astrazenecaclinicaltrials.com/ (accessed 25 November 2012).
-
- Borgstrom L, Asking L, Olsson H, Peterson S. Lack of interaction between disease severity and therapeutic response with budesonide/formoterol in a single inhaler [Abstract]. American Thoracic Society 100th International Conference; 2004 May 21‐26; Orlando, C22 Poster 505.
-
- Calverley P, Pauwels Dagger R, Lofdahl CG, Svensson K, Higenbottam T, Carlsson LG, et al. Relationship between respiratory symptoms and medical treatment in exacerbations of COPD. European Respiratory Journal 2005;26(3):406‐13. [0903‐1936] - PubMed
-
- Calverley PM, Szafranski W, Andersson. Budesonide/formoterol is a well‐tolerated long term maintenance therapy for COPD [Abstract]. European Respiratory Journal 2005;26(Suppl 49):Abstract 1917.
Tashkin 2008 [SHINE] {published data only}
-
- AstraZeneca. A 6‐month double‐blind, double‐dummy, randomized, parallel group, multicenter efficacy & safety study of SYMBICORT® pMDI 2 x 160/4.5mg & 80/4.5mg bid compared to formoterol TBH, budesonide pMDI (& the combination) & placebo in COPD patients. D5899C00002. http://www.astrazenecaclinicaltrials.com/ (accessed23 November 2012).
-
- Bleecker ER, Meyers DA, Bailey WC, Sims AM, Bujac SR, Goldman M, et al. Effect of β2‐adrenergic receptor gene polymorphism Gly16Arg on response to budesonide/formoterol pressurized metered‐dose inhaler in chronic obstructive pulmonary disease [Abstract]. American Journal of Respiratory and Critical Care Medicine 2011;183:A4086.
-
- Celli BR, Tashkin DP, Rennard SI, McElhattan J, Martin UJ. Bronchodilator responsiveness and onset of effect with budesonide/formoterol pMDI in COPD. Respiratory Medicine 2011;105(8):1176‐88. - PubMed
-
- Nelson HS, Tashkin DP, Rennard SI, Martin P, Goldman M, Silkoff PE. Onset of bronchodilation with budesonide and formoterol administered in one pressurized metered‐dose inhaler in patients with moderate to very severe chronic obstructive pulmonary disease [Abstract]. Chest 2008;134(4):105002s. - PubMed
-
- Tashkin DP, Rennard SI, Martin P, Goldman M, Silkoff PE. Efficacy of budesonide/formoterol administered via one pressurized metered‐dose inhaler over 6 months in patients with chronic obstructive pulmonary disease [Abstract]. Chest 2008;134(4):105001s.
Tashkin 2012 {published data only}
-
- Doherty D, Tashkin D, Kerwin E, Eduardo Matiz‐Bueno C, Shekar T, Banerjee S, et al. Efficacy and safety of mometasone furoate/formoterol in subjects with moderate to very severe chronic obstructive pulmonary disease: results from two phase three 26‐week trials [Abstract]. Chest 2011;140(4):535A.
-
- Kerwin E, Tashkin D, Matiz‐Bueno CE, Doherty D, Shekar T, Banerjee S, et al. Quality of life following 26 weeks of mometasone furoate/formoterol therapy: results from two phase three trials in subjects with moderate to very severe chronic obstructive pulmonary disease [Abstract]. Chest 2011;140(4):558A.
-
- Matiz‐Bueno CE, Doherty D, Kerwin E, Tashkin D, Shekar T, Banerjee S, et al. The long‐term safety characteristics of mometasone furoate/formoterol for the treatment of moderate to very severe chronic obstructive pulmonary disease: pooled findings from two 1‐year multicenter clinical trials [Abstract]. Chest 2011;140(4):548A.
-
- Tashkin D, Doherty D, Kerwin E, Matiz‐Bueno CE, Shekar T, Banerjee S, et al. The effect of mometasone furoate/formoterol combination therapy on chronic obstructive pulmonary disease (COPD) exacerbations: results from two phase three trials in subjects with moderate to very severe COPD [Abstract]. Chest 2011;140(4):549A.
-
- Tashkin DP, Doherty DE, Kerwin E, Matiz‐Bueno CE, Knorr B, Shekar T, et al. Efficacy and safety characteristics of mometasone furoate/formoterol fumarate fixed‐dose combination in subjects with moderate to very severe COPD: findings from pooled analysis of two randomized, 52‐week placebo‐controlled trials. International Journal of COPD 2012;7:73‐86. - PMC - PubMed
Vogelmeier 2008 {published data only}
-
- Arievich H, Potena A, Fonay K, Vogelmeier CF, Overend T, Smith J, et al. Formoterol given either alone or together with tiotropium reduces the rate of exacerbations in stable COPD patients [Abstract]. European Respiratory Journal 2006;28(Suppl 50):440s [P2514].
-
- Vogelmeier C, Kardos P, Harari S, Gans SJ, Stenglein S, Thirlwell J. Formoterol mono‐ and combination therapy with tiotropium in patients with COPD: a 6‐month study. Respiratory Medicine 2008;102(11):1511‐20. - PubMed
-
- Vogelmeier CF, Harari SA, Fonay K, Beier J, Overend T, Till D, et al. Formoterol and tiotropium both improve lung function in stable COPD patients with some additional benefit when given together [Abstract]. European Respiratory Journal 2006;28(Suppl 50):429s [P2506].
Wadbo 2002 {published data only}
-
- Wadbo M, Lofdahl CG, Larsson K, Skoogh BE, Tornling G, Arwestrom E, et al. Effects of formoterol and ipratropium bromide in COPD: a 3‐month placebo‐controlled study. European Respiratory Journal 2002;20(5):1138‐46. [0903‐1936] - PubMed
Watkins 2002 {published data only}
-
- Watkins M, Wire P, Yates J, Fischer T, Chang C, Horstman D, et al. Sustained Fev increases in COPD patients induced by Salmeterol 50 mcg twice daily via the diskus inhaler. American Journal of Respiratory and Critical Care Medicine 2002;165(Suppl 8):A228. - PubMed
References to studies excluded from this review
Boyd 1995 {published data only}
-
- Boyd G, Crawford C. Salmeterol (SALM) for treatment of patients with chronic obstructive pulmonary disease (COPD). European Respiratory Journal 1995;8(Suppl 19):167S. - PubMed
-
- Boyd G, Morice AH, Pounsford JC, Siebert M, Peslis N, Crawford C. An evaluation of salmeterol in the treatment of chronic obstructive pulmonary disease (COPD) [published erratum appears in European Respiratory Journal 1997 Jul;10(7):1696]. European Respiratory Journal 1997;10(4):815‐21. - PubMed
-
- GlaxoSmithKline. A multicentre, randomised, double‐blind, parallel group study to compare the efficacy and safety of inhaled salmeterol xinafoate 50 microg bd and inhaled salmeterol xinafoate 100 microg bd with placebo, all administered via the metered‐dose inhaler, in the treatment of patients with chronic obstructive pulmonary disease. www.gsk‐clinicalstudyregister.com (accessed 11 December 2012).
-
- Jones PW, Bosh TK. Quality of life changes in COPD patients treated with salmeterol. American Journal of Respiratory and Critical Care Medicine 1997;155(4):1283‐9. - PubMed
-
- Jones PW, Wilson K, Sondhi S. Cost‐effectiveness of salmeterol in patients with chronic obstructive pulmonary disease: An economic evaluation. Respiratory Medicine 2003;97(1):20‐6. [0954‐6111] - PubMed
Celli 2003 {published data only}
-
- Celli B, Halpin D, Hepburn R, Byrne N, Keating ET, Goldman M. Symptoms are an important outcome in chronic obstructive pulmonary disease clinical trials: results of a 3‐month comparative study using the Breathlessness, Cough and Sputum Scale (BCSS). Respiratory Medicine 2003;97(Suppl A):S35‐43. [0954‐6111] - PubMed
Chapman 2002 {published data only}
-
- Chapman K, Kuipers AF, Goldstein R, James MH. The addition of salmeterol 50mcg bid to anticholinergic treatment in COPD [Abstract]. American Journal of Respiratory and Critical Care Medicine 1999;159(3):A523.
-
- Chapman KR, Arvidsson P, Chuchalin AG, Dhillon DP, Faurschou P, Goldstein RS, et al. The addition of salmeterol 50 microg bid to anticholinergic treatment in patients with COPD: a randomized, placebo controlled trial. Chronic obstructive pulmonary disease. Canadian Respiratory Journal 2002;9(3):178‐85. [1198‐2241] - PubMed
-
- GlaxoSmithKline. A multi‐centre, double‐blind, parallel group study to evaluate the efficacy of SEREVENT 50 microg BID versus placebo BID all administered via the multi dose powder inhaler (DISKUS/ACCUHALERÆË) in terms of symptoms in the treatment of patients with chronic obstructive pulmonary disease. www.gsk‐clinicalstudyregister.com (accessed 15 December 2012).
Dal Negro 2003 {published data only}
-
- Dal Negro R, Micheletto C, Trevisan F, Tognella S, Pomari C. Salmeterol & fluticasone 50 µg/250 µg bid vs salmeterol 50µg bid and vs placebo in the long‐term treatment of COPD. American Journal of Respiratory and Critical Care Medicine 2002;165(Suppl 8):A228.
-
- Dal Negro RW, Pomari C, Tognella S, Micheletto C. Salmeterol & fluticasone 50 mug/250 mug bid in combination provides a better long‐term control than salmeterol 50 mug bid alone and placebo in COPD patients already treated with theophylline. Pulmonary Pharmacology and Therapeutics 2003;16(4):241‐6. - PubMed
Rutten‐van Molken 1999 {published data only}
-
- GlaxoSmithKline. Long‐term treatment of chronic obstructive pulmonary disease with salmeterol and the additive effect of ipratropium. www.gsk‐clinicalstudyregister.com (accessed 8 December 2012).
-
- Stahl E, Wadbo M, Bengtsson T, Strom K, Lofdahl CG. Health‐related quality of life, symptoms, exercise capacity and lung function during treatment for moderate to severe COPD. Journal of Outcomes Research 2001;5:11‐24.
-
- Noord JA, Munck D, Bantje TA, Hop WCJ, Bommer AM. Efficacy and safety of salmeterol (SLM) and ipratropium bromide (IPB) in patients with chronic obstructive pulmonary disease (COPD). American Journal of Respiratory and Critical Care Medicine 1998;157(Suppl 3):A799.
-
- Noord JA, Munck DR, Bantje TA, Hop WC, Akveld ML, Bommer AM. Long‐term treatment of chronic obstructive pulmonary disease with salmeterol and the additive effect of ipratropium. European Respiratory Journal 2000;15(5):878‐85. - PubMed
Steffensen 1996 {published data only}
-
- Steffensen I, Faurschou P, Riska H, Rostrup J, Wegener T. Inhaled formoterol dry powder in the treatment of patients with reversible obstructive airway disease. A 3‐month, placebo‐controlled comparison of the efficacy and safety of formoterol and salbutamol, followed by a 12‐month trial with formoterol. Allergy 1995;50(8):657‐63. [0105‐4538: (Print)] - PubMed
-
- Steffensen IE, Faurschou P. Formoterol as inhalation powder in the treatment of patients with reversible obstructive lung diseases. A 3‐month placebo‐controlled comparison of the effects of formoterol and salbutamol, followed by a 12‐month period with formoterol alone. Ugeskr‐Laeger 1996;158(49):7092‐6. - PubMed
Stockley 2006 {published data only}
-
- GlaxoSmithKline. A multi‐centre, randomised, double‐blind, placebo‐controlled, parallel‐group study to investigate the effect of 12 months treatment with salmeterol (50mcg bd), delivered via the DISKUS* inhaler, on the incidence of moderate and severe exacerbations in subjects with chronic obstructive pulmonary disease (COPD) when added to their usual treatment regimen. www.gsk‐clinicalstudyregister.com (accessed 8 December 2012).
-
- Stockley R, Davis EA, Sondhi S, Rice L. [Salmeterol provides sustained health status improvement over 12 months in patients with COPD [abstract]]. European Respiratory Society Annual Congress; 2002 Sep 14‐18; Stockholm, Abstract P1567.
-
- Stockley R, Rice L, Chopra N. [Salmeterol added to usual therapy gives a rapid improvement in lung function that is sustained at 6 and 12 months in patients with COPD [abstract]]. American Thoracic Society 99th International Conference; 2003 May 16‐21; Seattle, A035 Poster D71.
-
- Stockley R, Rice L, Chopra N. [Salmeterol added to usual therapy significantly reduces the frequency of moderate/severe exacerbations in patients with COPD [abstract]]. American Thoracic Society 99th International Conference; 2003 May 16‐21; Seattle, A108 Poster C51.
-
- Stockley RA, Chopra N. [Salmeterol, added to usual therapy, is an effective bronchodilator over 12 months of treatment in chronic obstructive pulmonary disease (COPD) [abstract]]. European Respiratory Society Annual Congress; 2002 Sep 14‐18; Stockholm, Abstract P1568.
References to studies awaiting assessment
Fattore 2005 {published data only}
-
- Fattore G, Torbica A, Mangone M. Cost‐analysis of four treatment strategies in the management of moderate‐to‐severe COPD: an application of non‐parametric bootstrap. [Italian]. Pharmacoeconomics Italian Research Articles 2005; Vol. 7, issue 2:135‐43. [1590‐9158]
References to ongoing studies
NCT01437397 {unpublished data only}
-
- NCT01437397. Efficacy, safety and tolerability of aclidinium bromide/formoterol fumarate compared with formoterol fumarate in patients with moderate to severe chronic obstructive pulmonary disease (COPD) (LAC). clinicaltrials.gov (accessed 30 July 2013).
NCT01572792 {unpublished data only}
-
- NCT01572792. Efficacy, safety and tolerability of two fixed dose combinations of aclidinium bromide/formoterol fumarate, aclidinium bromide, formoterol fumarate and placebo for 28‐weeks treatment in patients with moderate to severe, stable chronic obstructive pulmonary disease (COPD). clinicaltrials.gov (accessed 30 July 2013).
Additional references
Appleton 2006
AstraZeneca
-
- AstraZeneca. AstraZeneca clinical trials. http://www.astrazenecaclinicaltrials.com/ (accessed 15 November 2012).
ATS/ERS 2011
-
- Qaseem A, Wilt TJ, Weinberger SE, Hanania NA, Criner G, Molen T, et al. Diagnosis and management of stable chronic obstructive pulmonary disease: a clinical practice guideline update from the American College of Physicians, American College of Chest Physicians, American Thoracic Society, and European Respiratory Society. Annals of Internal Medicine 2011;155(3):179‐91. - PubMed
Beeh 2010
-
- Beeh KM, Beier J. The short, the long and the "ultra‐long": why duration of bronchodilator action matters in chronic obstructive pulmonary disease. Advances in Therapy 2010;27(3):150‐9. - PubMed
Berger 2008
-
- Berger WE, Nadel JA. Efficacy and safety of formoterol for the treatment of chronic obstructive pulmonary disease. Respiratory Medicine 2008;102(2):173‐88. - PubMed
BNF 2009
-
- British Medical Association and the Royal Pharmaceutical Society of Great Britain. British National Formulary. British National Formulary. 8th Edition. UK: BMJ Publishing Group, 2009.
Calverley 2003
-
- Calverley PMA, Spencer S, Willits L, Burge S, Jones P. Withdrawal from treatment as an outcome in the ISOLDE study of COPD. Chest 2003;124:1350‐6. - PubMed
Cheyne 2012
-
- Cheyne L, Irvin‐Sellers MJ, White J. Tiotropium versus ipratropium bromide for chronic obstructive pulmonary disease. Cochrane Database of Systematic Reviews 2012, Issue 1. [DOI: 10.1002/14651858.CD009552] - DOI
Chong 2011
Chong 2012
Effing 2007
Geake 2012
GlaxoSmithKline
-
- GlaxoSmithKline. GSK clinical study register. http://www.gsk‐clinicalstudyregister.com/ (accessed 15 November 2012).
GOLD 2013
-
- Global Initiative for Chronic Obstructive Lung Disease. From the Global Strategy for the Diagnosis, Management and Prevention of COPD, Global Initiative for Chronic Obstructive Lung Disease (GOLD) 2013. http://www.goldcopd.com (accessed 9/10/13).
Higgins 2009
-
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011 ]. The Cochrane Collaboration, www.cochrane‐handbook.org, 2009.
Hutchinson 2010
-
- Hutchinson A, Brand C, Irving L, Roberts C, Thompson P, Campbell D. Acute care costs of patients admitted for management of chronic obstructive pulmonary disease exacerbations: contribution of disease severity, infection and chronic heart failure. Internal Medicine Journal 2010;40(5):364‐71. - PubMed
Karner 2011
Karner 2011a
Karner 2012
Karner 2012a
Karner 2012b
Kliber 2010
Lacasse 2006
Nannini 2010
Nannini 2010a
Nannini 2012
-
- Nannini LJ, Lasserson TJ, Poole P. Combined corticosteroid and long‐acting beta2‐agonist in one inhaler versus long‐acting beta2‐agonists for chronic obstructive pulmonary disease. Cochrane Database of Systematic Reviews 2012, Issue 9. [DOI: 10.1002/14651858.CD006829.pub2] - DOI
NICE 2010
-
- National Institute for Health and Clinical Excellence. Chronic obstructive pulmonary disease, Costing report, Implementing NICE guidance. http://guidance.nice.org.uk/CG101/CostingReport/pdf/English (accessed 17 December 2012).
NICE 2011
-
- National Institute for Health and Clinical Excellence. CG101 Chronic obstructive pulmonary disease (update): NICE guideline. http://guidance.nice.org.uk/CG101/NICEGuidance/pdf/English (accessed 17 November 2012).
Puhan 2011
Rodrigo 2008
-
- Rodrigo GJ, Nannini LJ, Rodriguez‐Roisin R. Safety of long‐acting β‐agonists in stable COPD: a systematic review. Chest 2008;133(5):1079‐87. - PubMed
Song 2010
Spencer 2011
Stockley 2006a
van der Meer 2001
Wang 2012
-
- Wang J, Nie B, Xiong W, Xu Y. Effect of long‐acting beta‐agonists on the frequency of COPD exacerbations: a meta‐analysis. Journal of Clinical Pharmacy and Therapeutics 2012;37(2):204‐11. [1365‐2710: (Electronic)] - PubMed
Welsh 2011
-
- Welsh EJ, Cates CJ, Poole P. Combination inhaled steroid and long‐acting beta2‐agonist versus tiotropium for chronic obstructive pulmonary disease. Cochrane Database of Systematic Reviews 2011, Issue 7. [DOI: 10.1002/14651858.CD007891.pub2] - DOI
WHO
-
- World Health Organization. Chronic respiratory diseases. www.who.int (accessed 20 November 2012).
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

