Transcription repressor Bach2 is required for pulmonary surfactant homeostasis and alveolar macrophage function
- PMID: 24127487
- PMCID: PMC3804940
- DOI: 10.1084/jem.20130028
Transcription repressor Bach2 is required for pulmonary surfactant homeostasis and alveolar macrophage function
Abstract
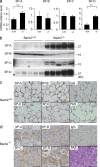
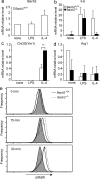
Pulmonary alveolar proteinosis (PAP) results from a dysfunction of alveolar macrophages (AMs), chiefly due to disruptions in the signaling of granulocyte macrophage colony-stimulating factor (GM-CSF). We found that mice deficient for the B lymphoid transcription repressor BTB and CNC homology 2 (Bach2) developed PAP-like accumulation of surfactant proteins in the lungs. Bach2 was expressed in AMs, and Bach2-deficient AMs showed alterations in lipid handling in comparison with wild-type (WT) cells. Although Bach2-deficient AMs showed a normal expression of the genes involved in the GM-CSF signaling, they showed an altered expression of the genes involved in chemotaxis, lipid metabolism, and alternative M2 macrophage activation with increased expression of Ym1 and arginase-1, and the M2 regulator Irf4. Peritoneal Bach2-deficient macrophages showed increased Ym1 expression when stimulated with interleukin-4. More eosinophils were present in the lung and peritoneal cavity of Bach2-deficient mice compared with WT mice. The PAP-like lesions in Bach2-deficient mice were relieved by WT bone marrow transplantation even after their development, confirming the hematopoietic origin of the lesions. These results indicate that Bach2 is required for the functional maturation of AMs and pulmonary homeostasis, independently of the GM-CSF signaling.
Figures








References
-
- Blanchard P.G., Festuccia W.T., Houde V.P., St-Pierre P., Brûlé S., Turcotte V., Côté M., Bellmann K., Marette A., Deshaies Y. 2012. Major involvement of mTOR in the PPARγ-induced stimulation of adipose tissue lipid uptake and fat accretion. J. Lipid Res. 53:1117–1125 10.1194/jlr.M021485 - DOI - PMC - PubMed
-
- Bonfield T.L., Farver C.F., Barna B.P., Malur A., Abraham S., Raychaudhuri B., Kavuru M.S., Thomassen M.J. 2003. Peroxisome proliferator-activated receptor-gamma is deficient in alveolar macrophages from patients with alveolar proteinosis. Am. J. Respir. Cell Mol. Biol. 29:677–682 10.1165/rcmb.2003-0148OC - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

