Clinical view on the importance of dendritic cells in asthma
- PMID: 24128155
- PMCID: PMC4479275
- DOI: 10.1586/1744666X.2013.837260
Clinical view on the importance of dendritic cells in asthma
Abstract
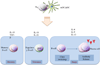
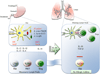
Allergic asthma is characterized by airway hyperresponsiveness and inflammation and may lead to airway remodeling in uncontrolled cases. Genetic predisposition to an atopic phenotype plays a major component in the pathophysiology of asthma. However, with tremendous role of epigenetic factors and environmental stimuli in precipitating an immune response, the underlying pathophysiological mechanisms are complicated. Dendritic cells are principal antigen-presenting cells and initiators of the immune response in allergic asthma. Their phenotype, guided by multiple factors may dictate the immune reaction to an allergic or tolerogenic response. Involvement of the local cytokine milieu, microbiome and interplay between immune cells add dimension to the fate of immune response. In addition to allergen exposure, these factors modulate DC phenotype and function. In this article, integration of many factors and pathways associated with the recruitment and activation of DCs in the pathophysiology of allergic asthma is presented in a clinical and translational manner.
Conflict of interest statement
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Figures





References
-
- Bharadwaj AS, Bewtra AK, Agrawal DK. Dendritic cells in allergic airway inflammation. Can. J. Physiol. Pharmacol. 2007;85(7):686–699. - PubMed
-
- Besnard A-G, Togbe D, Guillou N, Erard F, Quesniaux V, Ryffel B. IL-33-activated dendritic cells are critical for allergic airway inflammation. Eur. J. Immunol. 2011;41(6):1675–1686. - PubMed
-
- Eiwegger T, Akdis Ca. IL-33 links tissue cells, dendritic cells and Th2 cell development in a mouse model of asthma. Eur. J. Immunol. 2011;41(6):1535–1538. - PubMed
Website
-
- AllFam Database of Allergen Families. Medical University of Vienna; 2013. [Accessed 24th June 2013]. http://www.meduniwien.ac.at/allergens/allfam/chart.php?kingdom=All&expos....
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
