Dectin-2 sensing of house dust mite is critical for the initiation of airway inflammation
- PMID: 24129160
- PMCID: PMC3998635
- DOI: 10.1038/mi.2013.74
Dectin-2 sensing of house dust mite is critical for the initiation of airway inflammation
Abstract
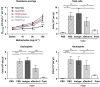
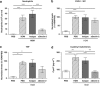
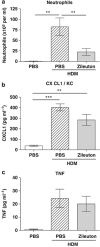
How the immune system senses aeroallergens and triggers an aberrant inflammation is poorly understood. Dectin-2 is a house dust mite (HDM)-sensing pattern recognition receptor. In a 3-week mouse model of repeated intranasal HDM challenge, anti-Dectin-2 potently attenuated the characteristic allergic inflammation and airway hyper-responsiveness. Anti-Dectin-2 also prevented neutrophil influx following a single HDM challenge. Interestingly, cysteinyl leukotrienes, but not chemokine and cytokine levels were inhibited by anti-Dectin-2 in this acute model, and in ex vivo challenge of cultured alveolar macrophages with HDM. Furthermore in the single-challenge model, zileuton, an inhibitor of leukotriene production, produced a similar effect as Dectin-2 blockade. Together these data suggest alveolar macrophage sensing of HDM by Dectin-2 elicits the production of cysteinyl leukotrienes, and this axis is key for the initiation of airway inflammation to this aeroallergen. Finally, we found Dectin-2-positive infiltrating cells present in bronchial biopsies from asthmatic subjects.
Figures



References
-
- Craig T.J. Aeroallergen sensitization in asthma: prevalence and correlation with severity. Allergy Asthma Proc. 2010;31:96–102. - PubMed
-
- Janeway C.A., Jr. Approaching the asymptote? Evolution and revolution in immunology. Cold Spring Harb Symp Quant Biol. 1989;54:1–13. - PubMed
-
- Svensson L., et al. House dust mite allergen activates human eosinophils via formyl peptide receptor and formyl peptide receptor-like 1. Eur J Immunol. 2007;37:1966–1977. - PubMed
-
- Asokananthan N., et al. House dust mite allergens induce proinflammatory cytokines from respiratory epithelial cells: the cysteine protease allergen, Der p 1, activates protease-activated receptor (PAR)-2 and inactivates PAR-1. J Immunol. 2002;169:4572–4578. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

