Silencing NOTCH signaling causes growth arrest in both breast cancer stem cells and breast cancer cells
- PMID: 24129237
- PMCID: PMC3833225
- DOI: 10.1038/bjc.2013.642
Silencing NOTCH signaling causes growth arrest in both breast cancer stem cells and breast cancer cells
Abstract
Background: Breast cancer stem cells (BCSCs) are characterized by high aldehyde dehydrogenase (ALDH) enzyme activity and are refractory to current treatment modalities, show a higher risk for metastasis, and influence the epithelial to mesenchymal transition (EMT), leading to a shorter time to recurrence and death. In this study, we focused on examination of the mechanism of action of a small herbal molecule, psoralidin (Pso) that has been shown to effectively suppress the growth of BSCSs and breast cancer cells (BCCs), in breast cancer (BC) models.
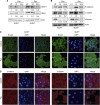
Methods: ALDH(-) and ALDH(+) BCCs were isolated from MDA-MB-231 cells, and the anticancer effects of Pso were measured using cell viability, apoptosis, colony formation, invasion, migration, mammosphere formation, immunofluorescence, and western blot analysis.
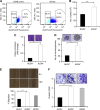
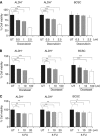
Results: Psoralidin significantly downregulated NOTCH1 signaling, and this downregulation resulted in growth inhibition and induction of apoptosis in both ALDH(-) and ALDH(+) cells. Molecularly, Pso inhibited NOTCH1 signaling, which facilitated inhibition of EMT markers (β-catenin and vimentin) and upregulated E-cadherin expression, resulting in reduced migration and invasion of both ALDH(-) and ALDH(+) cells.
Conclusion: Together, our results suggest that inhibition of NOTCH1 by Pso resulted in growth arrest and inhibition of EMT in BCSCs and BCCs. Psoralidin appears to be a novel agent that targets both BCSCs and BCCs.
Figures



References
-
- Al-Hussaini H, Subramanyam D, Reedijk M, Sridhar SS. Notch signaling pathway as a therapeutic target in breast cancer. Mol Cancer Ther. 2011;10 (1:9–15. - PubMed
-
- Alison MR, Lin WR, Lim SM, Nicholson LJ. Cancer stem cells: in the line of fire. Cancer Treat Rev. 2012;38 (6:589–598. - PubMed
-
- Charafe-Jauffret E, Ginestier C, Iovino F, Tarpin C, Diebel M, Esterni B, Houvenaeghel G, Extra JM, Bertucci F, Jacquemier J, Xerri L, Dontu G, Stassi G, Xiao Y, Barsky SH, Birnbaum D, Viens P, Wicha MS. Aldehyde dehydrogenase 1-positive cancer stem cells mediate metastasis and poor clinical outcome in inflammatory breast cancer. Clin Cancer Res. 2010;16 (1:45–55. - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

