Structural studies and investigation on the activity of imidazole-derived thiosemicarbazones and hydrazones against crop-related fungi
- PMID: 24129274
- PMCID: PMC6270485
- DOI: 10.3390/molecules181012645
Structural studies and investigation on the activity of imidazole-derived thiosemicarbazones and hydrazones against crop-related fungi
Abstract
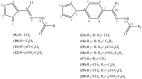
New imidazole derived thiosemicarbazones and hydrazones were prepared by condensation of 4(5)-imidazole carboxaldehyde, 4-(1H-imidazole-1-yl)benzaldehyde and 4-(1H-imidazole-1-yl)acetophenone with a thiosemicarbazide or hydrazide. All compounds were characterized by quantitative elemental analysis, IR and NMR techniques. Eight structures were determined by single crystal X-ray diffraction. The antifungal activities of the compounds were evaluated. None of the compounds exhibited significant activity against Aspergillus flavus and Candida albicans, while 4(5)-imidazolecarboxaldehyde thiosemicarbazone (ImT) and 4-(1H-imidazole-1-yl)benzaldehyde thiosemicabazone (4ImBzT) were highly and selectively active against Cladosporium cladosporioides. 4(5)-Imidazolecarboxaldehyde benzoyl hydrazone (4(5)ImPh), 4(5)-imidazolecarboxaldehyde-para-chlorobenzoyl hydrazone (4(5)ImpClPh), 4(5)-imidazolecarboxaldehyde-para-nitrobenzoyl hydrazone (4(5)ImpNO2Ph), 4-(imidazole-1-yl)acetophenone-para-chloro-benzoyl hydrazone (4ImAcpClPh) and 4-(imidazole-1-yl)acetophenone-para-nitro-benzoylhydrazone (4ImAcpNO2Ph) were highly active against Candida glabrata. 4(5)ImpClPh and 4(5)ImpNO2Ph were very effective against C. cladosporioides. In many cases, activity was superior to that of the reference compound nystatin.
Figures
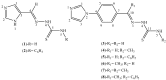

Similar articles
-
7-chloroquinolin-4-yl arylhydrazone derivatives: synthesis and antifungal activity.ScientificWorldJournal. 2011 Jul 28;11:1489-95. doi: 10.1100/tsw.2011.141. ScientificWorldJournal. 2011. PMID: 21805018 Free PMC article.
-
Two Vanadium(V) Complexes Derived from Bromo and Chloro-Substituted Hydrazone Ligands: Syntheses, Crystal Structures and Antimicrobial Property.Acta Chim Slov. 2020 Dec;67(4):1281-1289. Acta Chim Slov. 2020. PMID: 33533465
-
Synthesis and antimicrobial activity of some new hydrazone-bridged thiazole-pyrrole derivatives.J Enzyme Inhib Med Chem. 2013 Aug;28(4):830-5. doi: 10.3109/14756366.2012.688043. Epub 2012 May 31. J Enzyme Inhib Med Chem. 2013. PMID: 22651798
-
Synthesis, Structures, and Antibacterial Activities of Hydrazone Compounds Derived from 4-Dimethylaminobenzohydrazide.Acta Chim Slov. 2021 Sep;68(3):567-574. Acta Chim Slov. 2021. PMID: 34897529
-
Silver(I) complexes with chromone-derived hydrazones: investigation on the antimicrobial and cytotoxic effects.Biometals. 2017 Jun;30(3):379-392. doi: 10.1007/s10534-017-0013-2. Epub 2017 Apr 13. Biometals. 2017. PMID: 28409296
Cited by
-
Acylhydrazones and Their Biological Activity: A Review.Molecules. 2022 Dec 9;27(24):8719. doi: 10.3390/molecules27248719. Molecules. 2022. PMID: 36557851 Free PMC article. Review.
-
Transition Metal Complexes of Thiosemicarbazides, Thiocarbohydrazides, and Their Corresponding Carbazones with Cu(I), Cu(II), Co(II), Ni(II), Pd(II), and Ag(I)-A Review.Molecules. 2023 Feb 14;28(4):1808. doi: 10.3390/molecules28041808. Molecules. 2023. PMID: 36838796 Free PMC article. Review.
-
New Hydrazinothiazole Derivatives of Usnic Acid as Potent Tdp1 Inhibitors.Molecules. 2019 Oct 15;24(20):3711. doi: 10.3390/molecules24203711. Molecules. 2019. PMID: 31619021 Free PMC article.
-
New p-Substituted Salicylaldehyde Phenylhydrazone Derivatives: Synthesis, Characterization, and Antioxidant Activities.Sci Pharm. 2014 Jul 1;82(4):735-47. doi: 10.3797/scipharm.1405-04. Print 2014 Oct-Dec. Sci Pharm. 2014. PMID: 26279974 Free PMC article.
-
Tin thiocarbonohydrazone complexes: synthesis, crystal structures and biological evaluation.Toxicol Res (Camb). 2019 Aug 30;8(6):862-867. doi: 10.1039/c9tx00109c. eCollection 2019 Nov 1. Toxicol Res (Camb). 2019. PMID: 32206301 Free PMC article.
References
-
- Bell A.S. Triazole antifungals: Itraconazole (Sporanox), fluconazole (Diflucan), Voriconazole (Vfend) and fosfluconazole (Prodif) In: Jonhson D.S., Li J.J., editors. The Art of Drug Synthesis. John Wiley & Sons, Inc.; Hoboken, NJ, USA: 2007. pp. 72–73.
-
- Narasimhan B., Sharma D., Kumar P. Biological importance of the imidazole nucleus in the new millennium. Med. Chem. Res. 2011;20:1119–1140. doi: 10.1007/s00044-010-9472-5. - DOI
-
- Melander C., Cavanagh J., Ritchie D.F., Rogers S.A., Robert W. Inhibition and dispersion of biofilms in plants with imidazole-triazole derivatives. 2010077603. WIPO Patent. 2010 Jul 8;
-
- Dumeunier R., Lamberth C., Trah S. Imidazole derivatives. 2010102866. WIPO Patent. 2010 Sep 10;
-
- Takemoto J.Y., Bensaci M., De Lucca A.J., Cleveland T.E., Gandhi N.R., Skebba V.P. Inhibition of Fungi from Diseased Grape by Syringomycin E-Rhamnolipid Mixture. Am. J. Enol. Vitic. 2010;61:210–214.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

