Electrochemical enzyme-linked immunosorbent assay (ELISA) for α-fetoprotein based on glucose detection with multienzyme-nanoparticle amplification
- PMID: 24129276
- PMCID: PMC6270425
- DOI: 10.3390/molecules181012675
Electrochemical enzyme-linked immunosorbent assay (ELISA) for α-fetoprotein based on glucose detection with multienzyme-nanoparticle amplification
Abstract
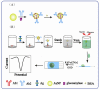
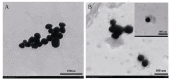
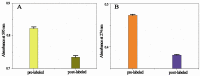
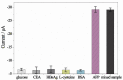
Since glucose biosensors are one of the most popular and widely used point-of-care testing devices, a novel electrochemical enzyme-linked immunosorbent assay (ELISA) for protein biomarkers has been developed based on a glucose detection strategy. In this study, α-fetoprotein (AFP) was used as the target protein. An electrochemical ELISA system was constructed using anti-AFP antibodies immobilized on microwell plates as the capture antibody (Ab1) and multi-label bioconjugates as signal tracer. The bioconjugates were synthesized by attaching glucoamylase and the secondary anti-AFP antibodies (Ab2) to gold nanoparticles (AuNPs). After formation of the sandwich complex, the Ab2-glucoamylase-AuNPs conjugates converted starch into glucose in the presence of AFP. The concentration of AFP can be calculated based on the linear relation between AFP and glucose, the concentration of which can be detected by the glucose biosensor. When the AFP concentration ranged from 0.05 to 100 ng/mL, a linear calibration plot (i (µA) = 13.62033 - 2.86252 logCAFP (ng/mL), r = 0.99886) with a detection limit of 0.02 ng/mL was obtained under optimal conditions. The electrochemical ELISA developed in this work shows acceptable stability and reproducibility, and the assay for AFP spiked in human serum also shows good recovery (97.0%-104%). This new method could be applied for detecting any protein biomarker with the corresponding antibodies.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

