Cost-effectiveness of newer antiretroviral drugs in treatment-experienced patients with multidrug-resistant HIV disease
- PMID: 24129369
- PMCID: PMC3932156
- DOI: 10.1097/QAI.0000000000000002
Cost-effectiveness of newer antiretroviral drugs in treatment-experienced patients with multidrug-resistant HIV disease
Abstract
Objective: Newer antiretroviral drugs provide substantial benefits but are expensive. The cost-effectiveness of using antiretroviral drugs in combination for patients with multidrug-resistant HIV disease was determined.
Design: A cohort state-transition model was built representing treatment-experienced patients with low CD4 counts, high viral load levels, and multidrug-resistant virus. The effectiveness of newer drugs (those approved in 2005 or later) was estimated from published randomized trials. Other parameters were estimated from a randomized trial and from the literature. The model had a lifetime time horizon and used the perspective of an ideal insurer in the United States. The interventions were combination antiretroviral therapy, consisting of 2 newer drugs and 1 conventional drug, compared with 3 conventional drugs. Outcome measures were life-years, quality-adjusted life-years (QALYs), costs, and incremental cost-effectiveness.
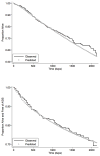
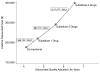
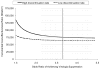
Results: Substituting newer antiretroviral drugs increased expected survival by 3.9 years in advanced HIV disease. The incremental cost-effectiveness ratio of newer, compared with conventional, antiretroviral drugs was $75,556/QALY gained. Sensitivity analyses showed that substituting only one newer antiretroviral drug cost $54,559 to $68,732/QALY, depending on assumptions about efficacy. Substituting 3 newer drugs cost $105,956 to $117,477/QALY. Cost-effectiveness ratios were higher if conventional drugs were not discontinued.
Conclusions: In treatment-experienced patients with advanced HIV disease, use of newer antiretroviral agents can be cost-effective, given a cost-effectiveness threshold in the range of $50,000 to $75,000 per QALY gained. Newer antiretroviral agents should be used in carefully selected patients for whom less expensive options are clearly inferior.
Figures
Similar articles
-
Use of genotypic resistance testing to guide hiv therapy: clinical impact and cost-effectiveness.Ann Intern Med. 2001 Mar 20;134(6):440-50. doi: 10.7326/0003-4819-134-6-200103200-00008. Ann Intern Med. 2001. PMID: 11255519
-
Cost effectiveness of darunavir/ritonavir 600/100 mg bid in treatment-experienced, lopinavir-naive, protease inhibitor-resistant, HIV-infected adults in Belgium, Italy, Sweden and the UK.Pharmacoeconomics. 2010;28 Suppl 1:147-67. doi: 10.2165/11587500-000000000-00000. Pharmacoeconomics. 2010. PMID: 21182349
-
US cost effectiveness of darunavir/ritonavir 600/100 mg bid in treatment-experienced, HIV-infected adults with evidence of protease inhibitor resistance included in the TITAN Trial.Pharmacoeconomics. 2010;28 Suppl 1:129-46. doi: 10.2165/11587490-000000000-00000. Pharmacoeconomics. 2010. PMID: 21182348
-
Study of the impact of HIV genotypic drug resistance testing on therapy efficacy.Verh K Acad Geneeskd Belg. 2001;63(5):447-73. Verh K Acad Geneeskd Belg. 2001. PMID: 11813503 Review.
-
Darunavir: a review of its use in the management of HIV infection in adults.Drugs. 2009;69(4):477-503. doi: 10.2165/00003495-200969040-00007. Drugs. 2009. PMID: 19323590 Review.
Cited by
-
The urgent need for newer drugs in routine HIV treatment in Africa: the case of Ghana.Front Epidemiol. 2025 Mar 14;5:1523109. doi: 10.3389/fepid.2025.1523109. eCollection 2025. Front Epidemiol. 2025. PMID: 40161547 Free PMC article. Review.
-
Estimating HIV Management and Comorbidity Costs Among Aging HIV Patients in the United States: A Systematic Review.J Manag Care Spec Pharm. 2020 Feb;26(2):104-116. doi: 10.18553/jmcp.2020.26.2.104. J Manag Care Spec Pharm. 2020. PMID: 32011956 Free PMC article.
-
The Cost-Effectiveness of Financial Incentives for Viral Suppression: HPTN 065 Study.Value Health. 2019 Feb;22(2):194-202. doi: 10.1016/j.jval.2018.09.001. Epub 2018 Nov 2. Value Health. 2019. PMID: 30711064 Free PMC article.
-
The Potential Cost-Effectiveness of Pre-Exposure Prophylaxis Combined with HIV Vaccines in the United States.Vaccines (Basel). 2017 May 24;5(2):13. doi: 10.3390/vaccines5020013. Vaccines (Basel). 2017. PMID: 28538691 Free PMC article.
-
The burden of chronic diseases and cost-of-care in subjects with HIV infection in a Health District of Northern Italy over a 12-year period compared to that of the general population.BMC Public Health. 2016 Nov 9;16(1):1146. doi: 10.1186/s12889-016-3804-4. BMC Public Health. 2016. PMID: 27829390 Free PMC article.
References
-
- Temesgen Z, Cainelli F, Poeschla EM, Vlahakis SA, Vento S. Approach to salvage antiretroviral therapy in heavily antiretroviral-experienced HIV-positive adults. Lancet Infect Dis. 2006;6:496–507. - PubMed
-
- Cozzi-Lepri A, Phillips AN, Ruiz L, Clotet B, Loveday C, Kjaer J, et al. Evolution of drug resistance in HIV-infected patients remaining on a virologically failing combination antiretroviral therapy regimen. AIDS. 2007;21:721–732. - PubMed
-
- Anis AH, Nosyk B, Sun H, Guh DP, Bansback N, Li X, et al. Quality of life of patients with advanced HIV/AIDS: measuring the impact of both AIDS-defining events and non-AIDS serious adverse events. J Acquir Immune Defic Syndr. 2009;51:631–639. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

