Compartmentalization of distinct cAMP signaling pathways in mammalian sperm
- PMID: 24129574
- PMCID: PMC3853279
- DOI: 10.1074/jbc.M113.489476
Compartmentalization of distinct cAMP signaling pathways in mammalian sperm
Abstract
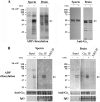
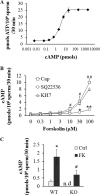
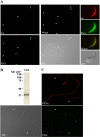
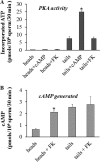
Fertilization competence is acquired in the female tract in a process known as capacitation. Capacitation is needed for the activation of motility (e.g. hyperactivation) and to prepare the sperm for an exocytotic process known as acrosome reaction. Although the HCO3(-)-dependent soluble adenylyl cyclase Adcy10 plays a role in motility, less is known about the source of cAMP in the sperm head. Transmembrane adenylyl cyclases (tmACs) are another possible source of cAMP. These enzymes are regulated by stimulatory heterotrimeric Gs proteins; however, the presence of Gs or tmACs in mammalian sperm has been controversial. In this study, we used Western blotting and cholera toxin-dependent ADP-ribosylation to show the Gs presence in the sperm head. Also, we showed that forskolin, a tmAC-specific activator, induces cAMP accumulation in sperm from both WT and Adcy10-null mice. This increase is blocked by the tmAC inhibitor SQ22536 but not by the Adcy10 inhibitor KH7. Although Gs immunoreactivity and tmAC activity are detected in the sperm head, PKA is only found in the tail, where Adcy10 was previously shown to reside. Consistent with an acrosomal localization, Gs reactivity is lost in acrosome-reacted sperm, and forskolin is able to increase intracellular Ca(2+) and induce the acrosome reaction. Altogether, these data suggest that cAMP pathways are compartmentalized in sperm, with Gs and tmAC in the head and Adcy10 and PKA in the flagellum.
Keywords: Acrosome Reaction; Adenylate Cyclase (Adenylyl Cyclase); Calcium Imaging; Cell Signaling; Cyclic AMP (cAMP); Forskolin; Heterotrimeric G Proteins; Protein Kinase A (PKA); Signal Transduction; Sperm Capacitation.
Figures



Similar articles
-
Bovine serum albumin-induced calcium influx triggers soluble adenylyl cyclase activation and cyclic AMP signalling pathways in mouse sperm capacitation.J Physiol. 2025 May;603(9):2633-2653. doi: 10.1113/JP288389. Epub 2025 May 5. J Physiol. 2025. PMID: 40320899
-
Extracellular cAMP activates molecular signalling pathways associated with sperm capacitation in bovines.Mol Hum Reprod. 2017 Aug 1;23(8):521-534. doi: 10.1093/molehr/gax030. Mol Hum Reprod. 2017. PMID: 28521061
-
Roles of intracellular cyclic AMP signal transduction in the capacitation and subsequent hyperactivation of mouse and boar spermatozoa.J Reprod Dev. 2013 Oct;59(5):421-30. doi: 10.1262/jrd.2013-056. J Reprod Dev. 2013. PMID: 24162806 Free PMC article. Review.
-
Extracellular regulation of sperm transmembrane adenylyl cyclase by a forward motility stimulating protein.PLoS One. 2014 Oct 28;9(10):e110669. doi: 10.1371/journal.pone.0110669. eCollection 2014. PLoS One. 2014. PMID: 25350397 Free PMC article.
-
First messenger regulation of capacitation via G protein-coupled mechanisms: a tale of serendipity and discovery.Mol Hum Reprod. 2003 Dec;9(12):739-48. doi: 10.1093/molehr/gag097. Mol Hum Reprod. 2003. PMID: 14614035 Review.
Cited by
-
Effects of 5-hydroxytryptamine on spermatozoal hyperactivation and in vitro fertilization in mice.J Reprod Dev. 2019 Dec 18;65(6):541-550. doi: 10.1262/jrd.2019-082. Epub 2019 Nov 6. J Reprod Dev. 2019. PMID: 31694987 Free PMC article.
-
Spermatozoal Mitochondrial Dynamics Markers and Other Functionality-Related Signaling Molecules Exert Circadian-like Response to Repeated Stress of Whole Organism.Cells. 2022 Mar 15;11(6):993. doi: 10.3390/cells11060993. Cells. 2022. PMID: 35326444 Free PMC article.
-
Serotonergic signals enhanced hamster sperm hyperactivation.J Reprod Dev. 2021 Aug 27;67(4):241-250. doi: 10.1262/jrd.2020-108. Epub 2021 May 12. J Reprod Dev. 2021. PMID: 33980767 Free PMC article.
-
Progesterone increases the success of in vitro fertilization via enhanced sperm hyperactivation in mice.J Reprod Dev. 2023 Jun 6;69(3):147-153. doi: 10.1262/jrd.2022-114. Epub 2023 Mar 18. J Reprod Dev. 2023. PMID: 36935121 Free PMC article.
-
Na+/H+ Exchangers (NHEs) in Mammalian Sperm: Essential Contributors to Male Fertility.Int J Mol Sci. 2023 Oct 7;24(19):14981. doi: 10.3390/ijms241914981. Int J Mol Sci. 2023. PMID: 37834431 Free PMC article. Review.
References
-
- Bailey J. L. (2010) Factors regulating sperm capacitation. Syst. Biol. Reprod. Med. 56, 334–348 - PubMed
-
- Visconti P. E., Galantino-Homer H., Moore G. D., Bailey J. L., Ning X., Fornes M., Kopf G. S. (1998) The molecular basis of sperm capacitation. J. Androl. 19, 242–248 - PubMed
-
- Morgan D. J., Weisenhaus M., Shum S., Su T., Zheng R., Zhang C., Shokat K. M., Hille B., Babcock D. F., McKnight G. S. (2008) Tissue-specific PKA inhibition using a chemical genetic approach and its application to studies on sperm capacitation. Proc. Natl. Acad. Sci. U.S.A. 105, 20740–20745 - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

