Antisense transcripts of the expanded C9ORF72 hexanucleotide repeat form nuclear RNA foci and undergo repeat-associated non-ATG translation in c9FTD/ALS
- PMID: 24129584
- PMCID: PMC3830741
- DOI: 10.1007/s00401-013-1192-8
Antisense transcripts of the expanded C9ORF72 hexanucleotide repeat form nuclear RNA foci and undergo repeat-associated non-ATG translation in c9FTD/ALS
Abstract
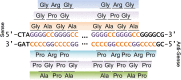
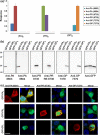
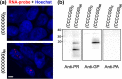
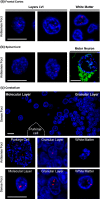
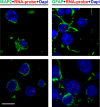
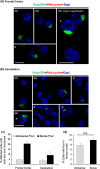
Frontotemporal dementia (FTD) and amyotrophic lateral sclerosis (ALS) are devastating neurodegenerative disorders with clinical, genetic, and neuropathological overlap. A hexanucleotide (GGGGCC) repeat expansion in a non-coding region of C9ORF72 is the major genetic cause of both diseases. The mechanisms by which this repeat expansion causes "c9FTD/ALS" are not definitively known, but RNA-mediated toxicity is a likely culprit. RNA transcripts of the expanded GGGGCC repeat form nuclear foci in c9FTD/ALS, and also undergo repeat-associated non-ATG (RAN) translation resulting in the production of three aggregation-prone proteins. The goal of this study was to examine whether antisense transcripts resulting from bidirectional transcription of the expanded repeat behave in a similar manner. We show that ectopic expression of (CCCCGG)66 in cultured cells results in foci formation. Using novel polyclonal antibodies for the detection of possible (CCCCGG)exp RAN proteins [poly(PR), poly(GP) and poly(PA)], we validated that (CCCCGG)66 is also subject to RAN translation in transfected cells. Of importance, foci composed of antisense transcripts are observed in the frontal cortex, spinal cord and cerebellum of c9FTD/ALS cases, and neuronal inclusions of poly(PR), poly(GP) and poly(PA) are present in various brain tissues in c9FTD/ALS, but not in other neurodegenerative diseases, including CAG repeat disorders. Of note, RNA foci and poly(GP) inclusions infrequently co-occur in the same cell, suggesting these events represent two distinct ways in which the C9ORF72 repeat expansion may evoke neurotoxic effects. These findings provide mechanistic insight into the pathogenesis of c9FTD/ALS, and have significant implications for therapeutic strategies.
Figures

Comment in
-
Making sense of the antisense transcripts in C9FTD/ALS.Acta Neuropathol. 2013 Dec;126(6):785-7. doi: 10.1007/s00401-013-1201-y. Acta Neuropathol. 2013. PMID: 24178412 Free PMC article. No abstract available.
References
-
- Al-Sarraj S, King A, Troakes C, Smith B, Maekawa S, Bodi I, Rogelj B, Al-Chalabi A, Hortobagyi T, Shaw CE. p62 positive, TDP-43 negative, neuronal cytoplasmic and intranuclear inclusions in the cerebellum and hippocampus define the pathology of C9orf72-linked FTLD and MND/ALS. Acta Neuropathol. 2011;122(6):691–702. doi: 10.1007/s00401-011-0911-2. - DOI - PubMed
-
- Almeida S, Gascon E, Tran H, Chou HJ, Gendron TF, Degroot S, Tapper AR, Sellier C, Charlet-Berguerand N, Karydas A, Seeley WW, Boxer AL, Petrucelli L, Miller BL, Gao FB. Modeling key pathological features of frontotemporal dementia with C9ORF72 repeat expansion in iPSC-derived human neurons. Acta Neuropathol. 2013;126(3):385–399. doi: 10.1007/s00401-013-1149-y. - DOI - PMC - PubMed
-
- Ash PE, Bieniek KF, Gendron TF, Caulfield T, Lin WL, Dejesus-Hernandez M, van Blitterswijk MM, Jansen-West K, Paul JW, 3rd, Rademakers R, Boylan KB, Dickson DW, Petrucelli L. Unconventional translation of C9ORF72 GGGGCC expansion generates insoluble polypeptides specific to c9FTD/ALS. Neuron. 2013;77(4):639–646. doi: 10.1016/j.neuron.2013.02.004. - DOI - PMC - PubMed
-
- Bieniek KF, Murray ME, Rutherford NJ, Castanedes-Casey M, Dejesus-Hernandez M, Liesinger AM, Baker MC, Boylan KB, Rademakers R, Dickson DW. Tau pathology in frontotemporal lobar degeneration with C9ORF72 hexanucleotide repeat expansion. Acta Neuropathol. 2013;125(2):289–302. doi: 10.1007/s00401-012-1048-7. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P50 AG016574/AG/NIA NIH HHS/United States
- R21 NS074121/NS/NINDS NIH HHS/United States
- R21 NS079807/NS/NINDS NIH HHS/United States
- R01 NS063964/NS/NINDS NIH HHS/United States
- P50 NS072187/NS/NINDS NIH HHS/United States
- R01 ES20395/ES/NIEHS NIH HHS/United States
- R21 NS084528/NS/NINDS NIH HHS/United States
- R01 ES020395/ES/NIEHS NIH HHS/United States
- P01 AG003949/AG/NIA NIH HHS/United States
- R01 NS080882/NS/NINDS NIH HHS/United States
- R01 AG026251/AG/NIA NIH HHS/United States
- R01 NS077402/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

