A comparative analysis of prognostic factor models for follicular lymphoma based on a phase III trial of CHOP-rituximab versus CHOP + 131iodine--tositumomab
- PMID: 24130072
- PMCID: PMC3872052
- DOI: 10.1158/1078-0432.CCR-13-1120
A comparative analysis of prognostic factor models for follicular lymphoma based on a phase III trial of CHOP-rituximab versus CHOP + 131iodine--tositumomab
Abstract
Purpose: There is currently no consensus on optimal frontline therapy for patients with follicular lymphoma. We analyzed a phase III randomized intergroup trial comparing six cycles of CHOP-R (cyclophosphamide-Adriamycin-vincristine-prednisone (Oncovin)-rituximab) with six cycles of CHOP followed by iodine-131 tositumomab radioimmunotherapy (RIT) to assess whether any subsets benefited more from one treatment or the other, and to compare three prognostic models.
Experimental design: We conducted univariate and multivariate Cox regression analyses of 532 patients enrolled on this trial and compared the prognostic value of the FLIPI (follicular lymphoma international prognostic index), FLIPI2, and LDH + β2M (lactate dehydrogenase + β2-microglobulin) models.
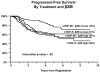
Results: Outcomes were excellent, but not statistically different between the two study arms [5-year progression-free survival (PFS) of 60% with CHOP-R and 66% with CHOP-RIT (P = 0.11); 5-year overall survival (OS) of 92% with CHOP-R and 86% with CHOP-RIT (P = 0.08); overall response rate of 84% for both arms]. The only factor found to potentially predict the impact of treatment was serum β2M; among patients with normal β2M, CHOP-RIT patients had better PFS compared with CHOP-R patients, whereas among patients with high serum β2M, PFS by arm was similar (interaction P value = 0.02).
Conclusions: All three prognostic models (FLIPI, FLIPI2, and LDH + β2M) predicted both PFS and OS well, though the LDH + β2M model is easiest to apply and identified an especially poor risk subset. In an exploratory analysis using the latter model, there was a statistically significant trend suggesting that low-risk patients had superior observed PFS if treated with CHOP-RIT, whereas high-risk patients had a better PFS with CHOP-R.
©2013 AACR.
Conflict of interest statement
Oliver W. Press (Principal Investigator) – consultancy with Roche/Genentech and clinical trial research funding from Roche/Genentech to the Fred Hutchinson Cancer Research Center. Joseph M. Unger – no conflict of interest. Lisa M. Rimsza – no conflict of interest. Jonathan W. Friedberg – consultancy with Genentech. Michael LeBlanc – no conflict of interest. Myron S. Czuczman - consultancy with Genentech Pharmaceuticals and Spectrum Pharmaceuticals. Mark S. Kaminski – research funding from GlaxoSmithKline; royalties from patents on CD20 Radioimmunotherapy. Rita M. Braziel – no conflict of interest. Catherine M. Spier – no conflict of interest. Ajay K. Gopal – consultancy with Seattle Genetics; honoraria from Seattle Genetics and Millennium/Takeda; research funding from Seattle Genetics, GlaxoSmithKline, Spectrum, Lilly, SBio, Piramal, Abbott, and Emergent Biosolutions. David G. Maloney – honoraria from Genentech, Roche, and GlaxoSmithKline; research funding from Genentech. Bruce D. Cheson – consultancy with Roche-Genentech. Shaker Dakhil – no conflict of interest. Thomas P. Miller – no conflict of interest. Richard I. Fisher – consultancy with Roche.
Figures



References
-
- Press OW. Follicular Lymphoma. In: Kaushansky K, Lichtman M, Beutler E, Kipps TJ, Seligsohn, Prchal, editors. Williams’ Hematology. 8. New York: McGraw-Hill, Medical Pub. Division; 2010. pp. 1565–74.
-
- Zelenetz AD, Abramson JS, Advani RH, Andreadis CB, Bartlett N, Bellam N, et al. Non-Hodgkin’s lymphomas. J Natl Compr Canc Netw. 2011;9:484–560. - PubMed
-
- Fisher RI, LeBlanc M, Press OW, Maloney DG, Unger JM, Miller TP. New treatment options have changed the survival of patients with follicular lymphoma. J Clin Oncol. 2005;23:8447–52. - PubMed
-
- Swenson WT, Wooldridge JE, Lynch CF, Forman-Hoffman VL, Chrischilles E, Link BK. Improved survival of follicular lymphoma patients in the United States. J Clin Oncol. 2005;23:5019–26. - PubMed
-
- Liu Q, Fayad L, Cabanillas F, Hagemeister FB, Ayers GD, Hess M, et al. Improvement of overall and failure-free survival in stage IV follicular lymphoma: 25 years of treatment experience at The University of Texas M.D. Anderson Cancer Center. J Clin Oncol. 2006;24:1582–9. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- N01 CA004919/CA/NCI NIH HHS/United States
- U10 CA012644/CA/NCI NIH HHS/United States
- CA35281/CA/NCI NIH HHS/United States
- CA76447/CA/NCI NIH HHS/United States
- NCIR01 CA076287/CA/NCI NIH HHS/United States
- CA37981/CA/NCI NIH HHS/United States
- U10 CA022433/CA/NCI NIH HHS/United States
- CA58416/CA/NCI NIH HHS/United States
- U10 CA027057/CA/NCI NIH HHS/United States
- N01 CA035176/CA/NCI NIH HHS/United States
- CA35261/CA/NCI NIH HHS/United States
- U10 CA037981/CA/NCI NIH HHS/United States
- U10 CA004919/CA/NCI NIH HHS/United States
- N01 CA035431/CA/NCI NIH HHS/United States
- U10 CA016385/CA/NCI NIH HHS/United States
- CA35128/CA/NCI NIH HHS/United States
- CA22433/CA/NCI NIH HHS/United States
- CA073590/CA/NCI NIH HHS/United States
- U10 CA045560/CA/NCI NIH HHS/United States
- CA12644/CA/NCI NIH HHS/United States
- U10 CA035128/CA/NCI NIH HHS/United States
- CA20319/CA/NCI NIH HHS/United States
- U10 CA076447/CA/NCI NIH HHS/United States
- N01 CA032102/CA/NCI NIH HHS/United States
- N01 CA013612/CA/NCI NIH HHS/United States
- U10 CA035192/CA/NCI NIH HHS/United States
- U10 CA045808/CA/NCI NIH HHS/United States
- U10 CA013612/CA/NCI NIH HHS/United States
- U10 CA031946/CA/NCI NIH HHS/United States
- U10 CA058416/CA/NCI NIH HHS/United States
- U10 CA014028/CA/NCI NIH HHS/United States
- N01 CA035119/CA/NCI NIH HHS/United States
- CA45808/CA/NCI NIH HHS/United States
- N01 CA046441/CA/NCI NIH HHS/United States
- R01 CA076287/CA/NCI NIH HHS/United States
- CA14028/CA/NCI NIH HHS/United States
- CA58882/CA/NCI NIH HHS/United States
- CA45377/CA/NCI NIH HHS/United States
- U10 CA035281/CA/NCI NIH HHS/United States
- CA11083/CA/NCI NIH HHS/United States
- CA58861/CA/NCI NIH HHS/United States
- CA35090/CA/NCI NIH HHS/United States
- N01 CA063844/CA/NCI NIH HHS/United States
- CA46282/CA/NCI NIH HHS/United States
- U10 CA035261/CA/NCI NIH HHS/United States
- U10 CA035178/CA/NCI NIH HHS/United States
- CA16385/CA/NCI NIH HHS/United States
- U10 CA045450/CA/NCI NIH HHS/United States
- U10 CA032102/CA/NCI NIH HHS/United States
- U10 CA046282/CA/NCI NIH HHS/United States
- CA45450/CA/NCI NIH HHS/United States
- CA67663/CA/NCI NIH HHS/United States
- N01 CA035178/CA/NCI NIH HHS/United States
- N01 CA038926/CA/NCI NIH HHS/United States
- U10 CA067575/CA/NCI NIH HHS/United States
- CA74811/CA/NCI NIH HHS/United States
- N01 CA027057/CA/NCI NIH HHS/United States
- U10 CA073590/CA/NCI NIH HHS/United States
- U10 CA046441/CA/NCI NIH HHS/United States
- U10 CA045377/CA/NCI NIH HHS/United States
- CA35192/CA/NCI NIH HHS/United States
- U10 CA058882/CA/NCI NIH HHS/United States
- U10 CA020319/CA/NCI NIH HHS/United States
- U10 CA038926/CA/NCI NIH HHS/United States
- U10 CA074811/CA/NCI NIH HHS/United States
- U10 CA035431/CA/NCI NIH HHS/United States
- U10 CA035119/CA/NCI NIH HHS/United States
- U10 CA011083/CA/NCI NIH HHS/United States
- CA52654/CA/NCI NIH HHS/United States
- U10 CA052654/CA/NCI NIH HHS/United States
- CA76429/CA/NCI NIH HHS/United States
- N01 CA067575/CA/NCI NIH HHS/United States
- U10 CA067663/CA/NCI NIH HHS/United States
- U10 CA035176/CA/NCI NIH HHS/United States
- U10 CA035090/CA/NCI NIH HHS/United States
- CA31946/CA/NCI NIH HHS/United States
- CA58723/CA/NCI NIH HHS/United States
- U10 CA063844/CA/NCI NIH HHS/United States
- U10 CA058861/CA/NCI NIH HHS/United States
- N01 CA045560/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

