Two actin-interacting protein 1 isoforms function redundantly in the somatic gonad and are essential for reproduction in Caenorhabditis elegans
- PMID: 24130131
- PMCID: PMC4082699
- DOI: 10.1002/cm.21152
Two actin-interacting protein 1 isoforms function redundantly in the somatic gonad and are essential for reproduction in Caenorhabditis elegans
Abstract
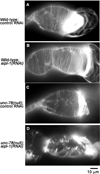
The somatic gonad of the nematode Caenorhabditis elegans exhibits highly regulated contractility during ovulation, which is essential for successful reproduction. Nonstriated actin filament networks in the myoepithelial sheath at the proximal ovary provide contractile forces to push a mature oocyte for ovulation, but the mechanism of assembly and regulation of the contractile actin networks is poorly understood. Here, we show that actin-interacting protein 1 (AIP1) is essential for the assembly of the contractile actin networks in the myoepithelial sheath. AIP1 promotes disassembly of actin filaments in the presence of actin depolymerizing factor (ADF)/cofilin. C. elegans has two AIP1 genes, unc-78 and aipl-1. Mutation or RNA interference of a single AIP1 isoform causes only minor impacts on reproduction. However, simultaneous depletion of the two AIP1 isoforms causes sterility. AIP1-depleted animals show very weak contractility of the myoepithelial sheath and fail to ovulate a mature oocyte, which results in accumulation of endomitotic oocytes in the ovary. Depletion of AIP1 prevents assembly of actin networks and causes abnormal aggregation of actin as well as ADF/cofilin in the myoepithelial sheath. These results indicate that two AIP1 isoforms have redundant roles in assembly of the contractile apparatuses necessary for C. elegans reproduction.
Keywords: actin depolymerizing factor/cofilin; actin dynamics; myoepithelial cells; ovulation; spermatheca; sterility.
Copyright © 2013 Wiley Periodicals, Inc.
Figures





References
-
- Aizawa H, Katadae M, Maruya M, Sameshima M, Murakami-Murofushi K, Yahara I. Hyperosmotic stress-induced reorganization of actin bundles in Dictyostelium cells over-expressing cofilin. Genes Cells. 1999;4:311–324. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

