Brief, instructional smokeless tobacco use among cigarette smokers who do not intend to quit: a pilot randomized clinical trial
- PMID: 24130144
- PMCID: PMC3954419
- DOI: 10.1093/ntr/ntt161
Brief, instructional smokeless tobacco use among cigarette smokers who do not intend to quit: a pilot randomized clinical trial
Abstract
Introduction: Low-nitrosamine smokeless tobacco (SLT) may have efficacy for smoking reduction and cessation, but its public health impact depends on how smokers use it.
Methods: This pilot study explored brief, instructional low-nitrosamine SLT use among smokers unmotivated to quit. Participants (N = 57) were randomized to either a free 2-week supply of Camel Snus group or a no-supply group. Of those randomized to use Camel Snus, half were told to use it to cope with smoking restrictions (Snus to Cope), and the remaining half were advised to use it to reduce smoking (Snus to Reduce). Participants were assessed before, during, and immediately after the intervention.
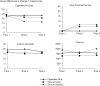
Results: Many Snus to Cope and Snus to Reduce participants reported daily use of Camel Snus, although the amount of use was low. Snus to Cope (18.4%) and Snus to Reduce (37.6%) participants reported a decline in number of cigarettes used per day, which was not reported by the control participants (p < .001). Intention to quit smoking and intention to quit all tobacco use (ps < .001) increased to a greater extent among Snus to Cope and Snus to Reduce participants than among control participants.
Conclusions: This study replicates previous work that shows that low-nitrosamine SLT use can lead to reduced smoking and increased intention to quit, and it adds direct evidence to suggest that the function of low-nitrosamine SLT use-either to cope with smoking restrictions or to reduce smoking-can have a differential impact on smoking behavior. Overall, the results highlight the importance of messaging and, more specifically, marketing of low-nitrosamine SLT to smokers.
Figures
References
-
- Bhattacharyya N. (2012). Trends in the use of smokeless tobacco in United States, 2000-2010. The Laryngoscope, 122, 2175–2178 doi:10.1002/lary.23448 - PubMed
-
- Biener L., Abrams D. B. (1991). The Contemplation Ladder: Validation of a measure of readiness to consider smoking cessation. Health Psychology, 10, 360–365 doi:10.1037/0022-006X.70.3.494 - PubMed
-
- Borland R., Yong H. H., Balmford J., Cooper J., Cummings K. M., O’Connor R. J, … Fong G. T. (2010). Motivational factors predict quit attempts but not maintenance of smoking cessation: Findings from the International Tobacco Control Four Country project. Nicotine and Tobacco Research, 12, S4–S11 doi:10.1093/ntr/ntq050 - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical