Expression of human butyrylcholinesterase with an engineered glycosylation profile resembling the plasma-derived orthologue
- PMID: 24130173
- PMCID: PMC3975692
- DOI: 10.1002/biot.201300229
Expression of human butyrylcholinesterase with an engineered glycosylation profile resembling the plasma-derived orthologue
Abstract
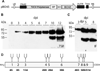
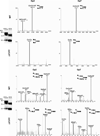
Human butyrylcholinesterase (BChE) is considered a candidate bioscavenger of nerve agents for use in pre- and post-exposure treatment. However, the presence and functional necessity of complex N-glycans (i.e. sialylated structures) is a challenging issue in respect to its recombinant expression. Here we transiently co-expressed BChE cDNA in the model plant Nicotiana benthamiana with vectors carrying the genes necessary for in planta protein sialylation. Site-specific sugar profiling of secreted recombinant BChE (rBChE) collected from the intercellular fluid revealed the presence of mono- and di-sialylated N-glycans, which largely resembles to the plasma-derived orthologue. Attempts to increase that sialylation content of rBChE by the over-expression of an additional glycosylation enzyme that generates branched N-glycans (i.e. β1,4-N-acetylglucosaminyl-transferase IV), allowed the production of rBChE decorated with tri-sialylated structures (up to 70%). Sialylated and non-sialylated plant-derived rBChE exhibited functional in vitro activity comparable to that of its commercially available equine-derived counterpart. These results demonstrate the ability of plants to generate valuable proteins with designed sialylated glycosylation profiles optimized for therapeutic efficacy. Moreover, the efficient synthesis of carbohydrates present only in minute amounts on the native protein (tri-sialylated N-glycans) facilitates the generation of a product with superior efficacies and/or new therapeutic functions.
Keywords: Butyrylcholinesterase; Glycoengineering; Plants; Recombinant biopharmaceuticals; Sialic acid.
Copyright © 2013 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
Conflict of interest statement
Figures


Similar articles
-
Oligomerization status influences subcellular deposition and glycosylation of recombinant butyrylcholinesterase in Nicotiana benthamiana.Plant Biotechnol J. 2014 Sep;12(7):832-9. doi: 10.1111/pbi.12184. Epub 2014 Mar 11. Plant Biotechnol J. 2014. PMID: 24618259 Free PMC article.
-
Generation of biologically active multi-sialylated recombinant human EPOFc in plants.PLoS One. 2013;8(1):e54836. doi: 10.1371/journal.pone.0054836. Epub 2013 Jan 25. PLoS One. 2013. PMID: 23372778 Free PMC article.
-
Engineering of sialylated mucin-type O-glycosylation in plants.J Biol Chem. 2012 Oct 19;287(43):36518-26. doi: 10.1074/jbc.M112.402685. Epub 2012 Sep 4. J Biol Chem. 2012. PMID: 22948156 Free PMC article.
-
Assessment of transient expression strategies to sialylate recombinant proteins in N. benthamiana.J Biotechnol. 2023 Mar 10;365:48-53. doi: 10.1016/j.jbiotec.2023.02.004. Epub 2023 Feb 15. J Biotechnol. 2023. PMID: 36805356 Review.
-
Glycoproteins from insect cells: sialylated or not?Biol Chem. 2001 Feb;382(2):151-9. doi: 10.1515/BC.2001.023. Biol Chem. 2001. PMID: 11308014 Free PMC article. Review.
Cited by
-
Creation of a protective pulmonary bioshield against inhaled organophosphates using an aerosolized bioscavenger.Ann N Y Acad Sci. 2016 Jun;1374(1):151-8. doi: 10.1111/nyas.13106. Epub 2016 Jul 2. Ann N Y Acad Sci. 2016. PMID: 27371808 Free PMC article. Review.
-
Implementation of Glycan Remodeling to Plant-Made Therapeutic Antibodies.Int J Mol Sci. 2018 Jan 31;19(2):421. doi: 10.3390/ijms19020421. Int J Mol Sci. 2018. PMID: 29385073 Free PMC article.
-
Treatment of acute organophosphate poisoning by using a cocaine hydrolase engineered from human butyrylcholinesterase.Chem Biol Interact. 2025 Aug 1;416:111552. doi: 10.1016/j.cbi.2025.111552. Epub 2025 May 8. Chem Biol Interact. 2025. PMID: 40339683
-
Rapid Transient Production of a Monoclonal Antibody Neutralizing the Porcine Epidemic Diarrhea Virus (PEDV) in Nicotiana benthamiana and Lactuca sativa.Planta Med. 2017 Dec;83(18):1412-1419. doi: 10.1055/s-0043-112344. Epub 2017 Jun 2. Planta Med. 2017. PMID: 28575911 Free PMC article.
-
Expression and glycoengineering of functionally active heteromultimeric IgM in plants.Proc Natl Acad Sci U S A. 2014 Apr 29;111(17):6263-8. doi: 10.1073/pnas.1320544111. Epub 2014 Mar 31. Proc Natl Acad Sci U S A. 2014. PMID: 24706782 Free PMC article.
References
-
- Doctor BP, Saxena A. Bioscavengers for the protection of humans against organophosphate toxicity. Chem.-Biol. Interact. 2005:167–171. - PubMed
-
- Mumford H, Docx CJ, Price ME, Green AC, et al. Human plasma-derived BuChE as a stoichiometric bioscavenger for treatment of nerve agent poisoning. Chem.-Biol. Interact. 2013;203:160–166. - PubMed
-
- Geyer BC, Kannan L, Cherni I, Woods RR, et al. Transgenic plants as a source for the bioscavenging enzyme, human butyrylcholinesterase. Plant Biotechnol. J. 2010;8:873–886. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

