Interferon regulatory factor-1 in flagellin-induced reprogramming: potential protective role of CXCL10 in cornea innate defense against Pseudomonas aeruginosa infection
- PMID: 24130180
- PMCID: PMC3832217
- DOI: 10.1167/iovs.13-12453
Interferon regulatory factor-1 in flagellin-induced reprogramming: potential protective role of CXCL10 in cornea innate defense against Pseudomonas aeruginosa infection
Abstract
Purpose: We previously showed that pre-exposure of the cornea to Toll-like receptor (TLR)5 ligand flagellin induces strong protective innate defense against microbial pathogens and hypothesized that flagellin modulates gene expression at the transcriptional levels. Thus, we sought to determine the role of one transcription factor, interferon regulatory factor (IRF1), and its target gene CXCL10 therein.
Methods: Superarray was used to identify transcription factors differentially expressed in Pseudomonas aeruginosa-challenged human corneal epithelial cells (CECs) with or without flagellin pretreatment. The expression of CXCL10, IRF1, LI-8(CXCL2), and IFNγ was determined by PCR, immunohistochemistry, Western/dot blotting, and/or ELISA. IRF1 knockout mice, CXCL10 and IFNγ neutralization, and NK cell depletion were used to define in vivo regulation and function of CXCL10. The severity of P. aeruginosa was assessed using clinical scoring, slit-lamp microscopy, bacterial counting, polymorphonuclear leukocytes (PMN) infiltration, and macrophage inflammatory protein 2/Chemokine (C-X-C motif) ligand 2 (MIP-2/CXCL2) expression.
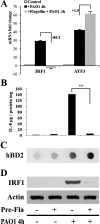
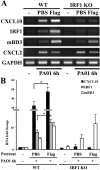
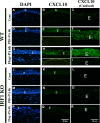
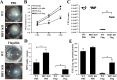
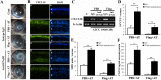
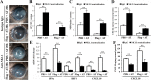
Results: Flagellin pretreatment drastically affected P. aeruginosa-induced IRF1 expression in human CECs. However, flagellin pretreatment augmented the P. aeruginosa-induced expression of Irf1 and its target gene Cxcl10 in B6 mouse corneas. Irf1 deficiency reduced infection-triggered CXCL10 expression, increased keratitis severity, and attenuated flagellin-elicited protection compared to values in wild-type (WT) controls. CXCL10 neutralization in the cornea of WT mice displayed pathogenesis similar to that of IRF1⁻/⁻ mice. IFNγ receptor neutralization and NK cell depletion prevented flagellin-augmented IRF1 and CXCL10 expression and increased the susceptibility to P. aeruginosa infection in mouse corneas.
Conclusions: IRF1 plays a role in the corneal innate immune response by regulating CXCL10 expression. IFNγ-producing NK cells augment the epithelial expression of IRF1 and CXCL10 and thus contribute to the innate defense of the cornea against P. aeruginosa infection.
Keywords: CXCL10; IRF1; cell reprogramming; inflammation; toll-like receptors.
Figures

Similar articles
-
ISG15 Acts as a Mediator of Innate Immune Response to Pseudomonas aeruginosa Infection in C57BL/6J Mouse Corneas.Invest Ophthalmol Vis Sci. 2020 May 11;61(5):26. doi: 10.1167/iovs.61.5.26. Invest Ophthalmol Vis Sci. 2020. PMID: 32416603 Free PMC article.
-
Inactivation of the miR-183/96/182 Cluster Decreases the Severity of Pseudomonas aeruginosa-Induced Keratitis.Invest Ophthalmol Vis Sci. 2016 Apr;57(4):1506-17. doi: 10.1167/iovs.16-19134. Invest Ophthalmol Vis Sci. 2016. PMID: 27035623 Free PMC article.
-
Protective efficacy of a peptide derived from a potential adhesin of Pseudomonas aeruginosa against corneal infection.Exp Eye Res. 2016 Feb;143:39-48. doi: 10.1016/j.exer.2015.10.011. Epub 2015 Oct 20. Exp Eye Res. 2016. PMID: 26500187
-
Corneal response to Pseudomonas aeruginosa infection.Prog Retin Eye Res. 2004 Jan;23(1):1-30. doi: 10.1016/j.preteyeres.2003.10.002. Prog Retin Eye Res. 2004. PMID: 14766315 Review.
-
Pseudomonas aeruginosa infection and inflammation during contact lens wear: a review.Optom Vis Sci. 2007 Apr;84(4):273-8. doi: 10.1097/OPX.0b013e3180439c3e. Optom Vis Sci. 2007. PMID: 17435510 Review.
Cited by
-
ISG15 in Host Defense Against Candida albicans Infection in a Mouse Model of Fungal Keratitis.Invest Ophthalmol Vis Sci. 2017 Jun 1;58(7):2948-2958. doi: 10.1167/iovs.17-21476. Invest Ophthalmol Vis Sci. 2017. PMID: 28599020 Free PMC article.
-
Matrix Metalloproteinase-13 as a Target for Suppressing Corneal Ulceration Caused by Pseudomonas aeruginosa Infection.J Infect Dis. 2015 Jul 1;212(1):116-27. doi: 10.1093/infdis/jiv016. Epub 2015 Jan 13. J Infect Dis. 2015. PMID: 25589337 Free PMC article.
-
Differentially Expressed Genes of Pseudomonas aeruginosa Isolates from Eyes with Keratitis and Healthy Conjunctival Sacs.Infect Drug Resist. 2022 Aug 12;15:4495-4506. doi: 10.2147/IDR.S374335. eCollection 2022. Infect Drug Resist. 2022. PMID: 35983295 Free PMC article.
-
The Role of Connexin-43 in the Inflammatory Process: A New Potential Therapy to Influence Keratitis.J Ophthalmol. 2019 Jan 21;2019:9312827. doi: 10.1155/2019/9312827. eCollection 2019. J Ophthalmol. 2019. PMID: 30805212 Free PMC article. Review.
-
IL-24 Promotes Pseudomonas aeruginosa Keratitis in C57BL/6 Mouse Corneas.J Immunol. 2017 May 1;198(9):3536-3547. doi: 10.4049/jimmunol.1602087. Epub 2017 Mar 22. J Immunol. 2017. PMID: 28330899 Free PMC article.
References
-
- Ueta M, Kinoshita S. Innate immunity of the ocular surface. Brain Res Bull. 2010; 81: 219–228 - PubMed
-
- Schaible B, Schaffer K, Taylor CT. Hypoxia, innate immunity and infection in the lung. Respir Physiol Neurobiol. 2010; 174: 235–243 - PubMed
-
- Kawai T, Akira S. Toll-like receptors and their crosstalk with other innate receptors in infection and immunity. Immunity. 2011; 34: 637–650 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

