Effect on postpartum hemorrhage of prophylactic oxytocin (10 IU) by injection by community health officers in Ghana: a community-based, cluster-randomized trial
- PMID: 24130463
- PMCID: PMC3794862
- DOI: 10.1371/journal.pmed.1001524
Effect on postpartum hemorrhage of prophylactic oxytocin (10 IU) by injection by community health officers in Ghana: a community-based, cluster-randomized trial
Abstract
Background: Oxytocin (10 IU) is the drug of choice for prevention of postpartum hemorrhage (PPH). Its use has generally been restricted to medically trained staff in health facilities. We assessed the effectiveness, safety, and feasibility of PPH prevention using oxytocin injected by peripheral health care providers without midwifery skills at home births.
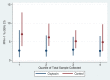
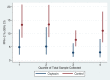
Methods and findings: This community-based, cluster-randomized trial was conducted in four rural districts in Ghana. We randomly allocated 54 community health officers (stratified on district and catchment area distance to a health facility: ≥10 km versus <10 km) to intervention (one injection of oxytocin [10 IU] one minute after birth) and control (no provision of prophylactic oxytocin) arms. Births attended by a community health officer constituted a cluster. Our primary outcome was PPH, using multiple definitions; (PPH-1) blood loss ≥500 mL; (PPH-2) PPH-1 plus women who received early treatment for PPH; and (PPH-3) PPH-2 plus any other women referred to hospital for postpartum bleeding. Unsafe practice is defined as oxytocin use before delivery of the baby. We enrolled 689 and 897 women, respectively, into oxytocin and control arms of the trial from April 2011 to November 2012. In oxytocin and control arms, respectively, PPH-1 rates were 2.6% versus 5.5% (RR: 0.49; 95% CI: 0.27-0.88); PPH-2 rates were 3.8% versus 10.8% (RR: 0.35; 95% CI: 0.18-0.63), and PPH-3 rates were similar to those of PPH-2. Compared to women in control clusters, those in the intervention clusters lost 45.1 mL (17.7-72.6) less blood. There were no cases of oxytocin use before delivery of the baby and no major adverse events requiring notification of the institutional review boards. Limitations include an unblinded trial and imbalanced numbers of participants, favoring controls.
Conclusion: Maternal health care planners can consider adapting this model to extend the use of oxytocin into peripheral settings including, in some contexts, home births.
Trial registration: ClinicalTrials.gov NCT01108289 Please see later in the article for the Editors' Summary.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
Comment in
-
The prevention of postpartum hemorrhage in the community.PLoS Med. 2013 Oct;10(10):e1001525. doi: 10.1371/journal.pmed.1001525. Epub 2013 Oct 1. PLoS Med. 2013. PMID: 24130464 Free PMC article.
References
-
- Khan KS, Wojdyla D, Say L, Gulmezoglu AM, Van Look PF (2006) WHO analysis of causes of maternal death: a systematic review. Lancet 367: 1066–1074. - PubMed
-
- World Health Organization, United Nations Children's Fund, United Nations Fund for Population Activities, The World Bank (2012) Trends in maternal mortality: 1990–2010. Geneva.
-
- United Nations Children's Fund (2000) State of the Word's Children. New York, UNICEF.
-
- United Nations Children's Fund (2012) State of the World's Children. New York, UNICEF.
-
- World Health Organization (2007) WHO recommendations for the prevention and treatment of postpartum haemorrhage. Geneva: Department of Reproductive Health, WHO. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous