Cortical plasticity within and across lifetimes: how can development inform us about phenotypic transformations?
- PMID: 24130524
- PMCID: PMC3793242
- DOI: 10.3389/fnhum.2013.00620
Cortical plasticity within and across lifetimes: how can development inform us about phenotypic transformations?
Abstract
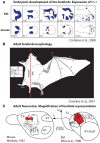
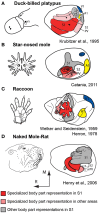
The neocortex is the part of the mammalian brain that is involved in perception, cognition, and volitional motor control. It is a highly dynamic structure that is dramatically altered within the lifetime of an animal and in different lineages throughout the course of evolution. These alterations account for the remarkable variations in behavior that species exhibit. Of particular interest is how these cortical phenotypes change within the lifetime of the individual and eventually evolve in species over time. Because we cannot study the evolution of the neocortex directly we use comparative analysis to appreciate the types of changes that have been made to the neocortex and the similarities that exist across taxa. Developmental studies inform us about how these phenotypic transitions may arise by alterations in developmental cascades or changes in the physical environment in which the brain develops. Both genes and the sensory environment contribute to aspects of the phenotype and similar features, such as the size of a cortical field, can be altered in a variety of ways. Although both genes and the laws of physics place constraints on the evolution of the neocortex, mammals have evolved a number of mechanisms that allow them to loosen these constraints and often alter the course of their own evolution.
Keywords: Evo-Devo; comparative neuroanatomy; cortical development; epigenetic; evolution.
Figures

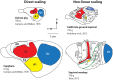
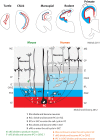
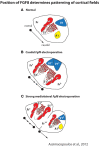
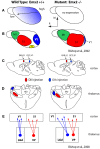


References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

