Biomarker-based prediction of progression in MCI: Comparison of AD signature and hippocampal volume with spinal fluid amyloid-β and tau
- PMID: 24130528
- PMCID: PMC3795312
- DOI: 10.3389/fnagi.2013.00055
Biomarker-based prediction of progression in MCI: Comparison of AD signature and hippocampal volume with spinal fluid amyloid-β and tau
Abstract
Objective: New diagnostic criteria for mild cognitive impairment (MCI) due to Alzheimer's disease (AD) have been developed using biomarkers aiming to establish whether the clinical syndrome is likely due to underlying AD. We investigated the utility of magnetic resonance imaging (MRI) and cerebrospinal fluid (CSF) biomarkers in predicting progression from amnesic MCI to dementia, testing the hypotheses that (1) markers of amyloid and neurodegeneration provide distinct and complementary prognostic information over different time intervals, and that (2) evidence of neurodegeneration in amyloid-negative MCI individuals would be useful prognostically.
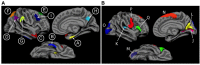
Methods: Data were obtained from the ADNI-1 (Alzheimer's Disease Neuroimaging Initiative Phase 1) database on all individuals with a baseline diagnosis of MCI, baseline MRI and CSF data, and at least one follow-up visit. MRI data were processed using a published set of a priori regions of interest to derive a measure known as the ``AD signature,'' as well as hippocampal volume. The CSF biomarkers amyloid-β, total tau, and phospho tau were also examined. We performed logistic regression analyses to identify the best baseline biomarker predictors of progression to dementia over 1 or 3 years, and Cox regression models to test the utility of these markers for predicting time-to-dementia.
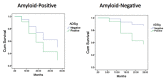
Results: For prediction of dementia in MCI, the AD signature cortical thickness biomarker performed better than hippocampal volume. Although CSF tau measures were better than CSF amyloid-β at predicting dementia within 1 year, the AD signature was better than all CSF measures at prediction over this relatively short-term interval. CSF amyloid-β was superior to tau and AD signature at predicting dementia over 3 years. When CSF amyloid-β was dichotomized using previously published cutoff values and treated as a categorical variable, a multivariate stepwise Cox regression model indicated that both the AD signature MRI marker and the categorical CSF amyloid-β marker were useful in predicting time-to-event diagnosis of AD dementia.
Conclusion: In amnesic MCI, short-term (1 year) prognosis of progression to dementia relates strongly to baseline markers of neurodegeneration, with the AD signature MRI biomarker of cortical thickness performing the best among MRI and CSF markers studied here. Longer-term (3 year) prognosis in these individuals was better predicted by a marker indicative of brain amyloid. Prediction of time-to-event in a survival model was predicted by the combination of these biomarkers. These results provide further support for emerging models of the temporal relationship of pathophysiologic events in AD and demonstrate the utility of these biomarkers at the prodromal stage of the illness.
Keywords: Alzheimer's disease; CSF biomarkers; MRI; biomarkers; mild cognitive impairment.
Figures

References
-
- Albert M. S., DeKosky S. T., Dickson D., Dubois B., Feldman H. H., Fox N. C., et al. (2011). The diagnosis of mild cognitive impairment due to Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 7 270–279 10.1016/j.jalz.2011.03.008 - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

