Alterations in muscle mass and contractile phenotype in response to unloading models: role of transcriptional/pretranslational mechanisms
- PMID: 24130531
- PMCID: PMC3795307
- DOI: 10.3389/fphys.2013.00284
Alterations in muscle mass and contractile phenotype in response to unloading models: role of transcriptional/pretranslational mechanisms
Abstract
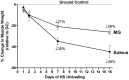
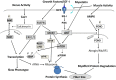
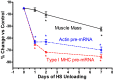
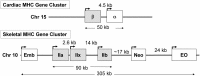
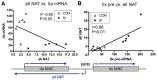
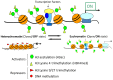
Skeletal muscle is the largest organ system in mammalian organisms providing postural control and movement patterns of varying intensity. Through evolution, skeletal muscle fibers have evolved into three phenotype clusters defined as a motor unit which consists of all muscle fibers innervated by a single motoneuron linking varying numbers of fibers of similar phenotype. This fundamental organization of the motor unit reflects the fact that there is a remarkable interdependence of gene regulation between the motoneurons and the muscle mainly via activity-dependent mechanisms. These fiber types can be classified via the primary type of myosin heavy chain (MHC) gene expressed in the motor unit. Four MHC gene encoded proteins have been identified in striated muscle: slow type I MHC and three fast MHC types, IIa, IIx, and IIb. These MHCs dictate the intrinsic contraction speed of the myofiber with the type I generating the slowest and IIb the fastest contractile speed. Over the last ~35 years, a large body of knowledge suggests that altered loading state cause both fiber atrophy/wasting and a slow to fast shift in the contractile phenotype in the target muscle(s). Hence, this review will examine findings from three different animal models of unloading: (1) space flight (SF), i.e., microgravity; (2) hindlimb suspension (HS), a procedure that chronically eliminates weight bearing of the lower limbs; and (3) spinal cord isolation (SI), a surgical procedure that eliminates neural activation of the motoneurons and associated muscles while maintaining neurotrophic motoneuron-muscle connectivity. The collective findings demonstrate: (1) all three models show a similar pattern of fiber atrophy with differences mainly in the magnitude and kinetics of alteration; (2) transcriptional/pretranslational processes play a major role in both the atrophy process and phenotype shifts; and (3) signaling pathways impacting these alterations appear to be similar in each of the models investigated.
Keywords: hindlimb suspension (unloading); myosin isoforms; non-coding RNAs; spaceflight; spinal cord isolation.
Figures

References
-
- Babij P., Booth F. W. (1988). Alpha-actin and cytochrome c mRNAs in atrophied adult rat skeletal muscle. Am. J. Physiol. Cell Physiol. 254, C651–C656 - PubMed
-
- Baldwin K. M., Herrick R. E., Ilyina-Kakeuva E., Oganov V. S. (1990). Effects of zero gravity on myofibril content and isomyosin distribution in rodent skeletal muscle. FASEB J. 4, 79–83 - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

