Tracheal epithelium cell volume responses to hyperosmolar, isosmolar and hypoosmolar solutions: relation to epithelium-derived relaxing factor (EpDRF) effects
- PMID: 24130533
- PMCID: PMC3795350
- DOI: 10.3389/fphys.2013.00287
Tracheal epithelium cell volume responses to hyperosmolar, isosmolar and hypoosmolar solutions: relation to epithelium-derived relaxing factor (EpDRF) effects
Abstract
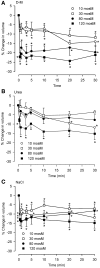
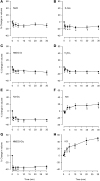
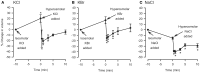
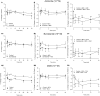
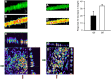
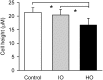
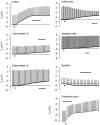
In asthmatic patients, inhalation of hyperosmolar saline or D-mannitol (D-M) elicits bronchoconstriction, but in healthy subjects exercise causes bronchodilation. Hyperventilation causes drying of airway surface liquid (ASL) and increases its osmolarity. Hyperosmolar challenge of airway epithelium releases epithelium-derived relaxing factor (EpDRF), which relaxes the airway smooth muscle. This pathway could be involved in exercise-induced bronchodilation. Little is known of ASL hyperosmolarity effects on epithelial function. We investigated the effects of osmolar challenge maneuvers on dispersed and adherent guinea-pig tracheal epithelial cells to examine the hypothesis that EpDRF-mediated relaxation is associated with epithelial cell shrinkage. Enzymatically-dispersed cells shrank when challenged with ≥10 mOsM added D-M, urea or NaCl with a concentration-dependence that mimics relaxation of the of isolated perfused tracheas (IPT). Cells shrank when incubated in isosmolar N-methyl-D-glucamine (NMDG) chloride, Na gluconate (Glu), NMDG-Glu, K-Glu and K2SO4, and swelled in isosmolar KBr and KCl. However, isosmolar challenge is not a strong stimulus of relaxation in IPTs. In previous studies amiloride and 4,4'-diisothiocyano-2,2'-stilbenedisulfonic acid (DIDS) inhibited relaxation of IPT to hyperosmolar challenge, but had little effect on shrinkage of dispersed cells. Confocal microscopy in tracheal segments showed that adherent epithelium is refractory to low hyperosmolar concentrations that induce dispersed cell shrinkage and relaxation of IPT. Except for gadolinium and erythro-9-(2-hydroxy-3-nonyl)adenine (EHNA), actin and microtubule inhibitors and membrane permeabilizing agents did not affect on ion transport by adherent epithelium or shrinkage responses of dispersed cells. Our studies dissociate relaxation of IPT from cell shrinkage after hyperosmolar challenge of airway epithelium.
Keywords: cell volume; epithelium-derived relaxing factor; exercise asthma.
Figures



References
LinkOut - more resources
Full Text Sources
Other Literature Sources

