Sensitive detection of measles virus infection in the blood and tissues of humanized mouse by one-step quantitative RT-PCR
- PMID: 24130556
- PMCID: PMC3795360
- DOI: 10.3389/fmicb.2013.00298
Sensitive detection of measles virus infection in the blood and tissues of humanized mouse by one-step quantitative RT-PCR
Abstract
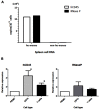
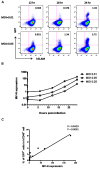
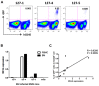
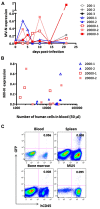
Live attenuated measles virus (MV) has long been recognized as a safe and effective vaccine, and it has served as the basis for development of various MV-based vaccines. However, because MV is a human-tropic virus, the evaluation of MV-based vaccines has been hampered by the lack of a small-animal model. The humanized mouse, a recently developed system in which an immunodeficient mouse is transplanted with human fetal tissues or hematopoietic stem cells, may represent a suitable model. Here, we developed a sensitive one-step quantitative reverse transcription (qRT)-PCR that simultaneously measures nucleocapsid (N) and human RNase P mRNA levels. The results can be used to monitor MV infection in a humanized mouse model. Using this method, we elucidated the replication kinetics of MV expressing enhanced green fluorescent protein both in vitro and in humanized mice in parallel with flow-cytometric analysis. Because our qRT-PCR system was sensitive enough to detect MV expression using RNA extracted from a small number of cells, it can be used to monitor MV infection in humanized mice by sequential blood sampling.
Keywords: EGFP expression; flow cytometry; humanized mouse; measles virus infection; quantitative RT-PCR.
Figures
References
-
- Brandler S., Marianneau P., Loth P., Lacote S., Combredet C., Frenkiel M. P., et al. (2012). Measles vaccine expressing the secreted form of West Nile virus envelope glycoprotein induces protective immunity in squirrel monkeys, a new model of West Nile virus infection. J. Infect. Dis. 206 212–219 10.1093/infdis/jis328 - DOI - PubMed
-
- Griffin D. E. (2007). “Measles virus,” in Fields Virology eds Knipe D. M., Howley P. M., Griffin D. E., Lamb R. A., Martin M. A., Roizman B., Straus S. E. 5 Edn (Philadelphia: Lippincott Williams & Wilkins, a Wolters Kluwer Business; ) 1551–1585
LinkOut - more resources
Full Text Sources
Other Literature Sources

