Can non-human primates serve as models for investigating dengue disease pathogenesis?
- PMID: 24130557
- PMCID: PMC3795305
- DOI: 10.3389/fmicb.2013.00305
Can non-human primates serve as models for investigating dengue disease pathogenesis?
Abstract
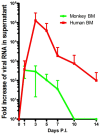
Dengue Virus (DV) infects between 50 and 100 million people globally, with public health costs totaling in the billions. It is the causative agent of dengue fever (DF) and dengue hemorrhagic fever/dengue shock syndrome (DHF/DSS), vector-borne diseases that initially predominated in the tropics. Due to the expansion of its mosquito vector, Aedes spp., DV is increasingly becoming a global problem. Infected individuals may present with a wide spectrum of symptoms, spanning from a mild febrile to a life-threatening illness, which may include thrombocytopenia, leucopenia, hepatomegaly, hemorrhaging, plasma leakage and shock. Deciphering the underlining mechanisms responsible for these symptoms has been hindered by the limited availability of animal models that can induce classic human pathology. Currently, several permissive non-human primate (NHP) species and mouse breeds susceptible to adapted DV strains are available. Though virus replication occurs in these animals, none of them recapitulate the cardinal features of human symptomatology, with disease only occasionally observed in NHPs. Recently our group established a DV serotype 2 intravenous infection model with the Indian rhesus macaque, which reliably produced cutaneous hemorrhages after primary virus exposure. Further manipulation of experimental parameters (virus strain, immune cell expansion, depletion, etc.) can refine this model and expand its relevance to human DF. Future goals include applying this model to elucidate the role of pre-existing immunity upon secondary infection and immunopathogenesis. Of note, virus titers in primates in vivo and in vitro, even with our model, have been consistently 1000-fold lower than those found in humans. We submit that an improved model, capable of demonstrating severe pathogenesis may only be achieved with higher virus loads. Nonetheless, our DV coagulopathy disease model is valuable for the study of select pathomechanisms and testing DV drug and vaccine candidates.
Keywords: bone marrow; dengue virus; disease pathogenesis; hemorrhage; non-human primate; platelet; platelet-lymphocyte aggregate; rhesus macaque.
Figures


References
-
- Ajariyakhajorn C., Mammen M. P., Jr., Endy T. P., Gettayacamin M., Nisalak A., Nimmannitiya S., et al. (2005). Randomized, placebo-controlled trial of nonpegylated and pegylated forms of recombinant human alpha interferon 2a for suppression of dengue virus viremia in rhesus monkeys. Antimicrobial Agents Chemother. 49, 4508–4514 10.1128/AAC.49.11.4508-4514.2005 - DOI - PMC - PubMed
-
- Anez G., Men R., Eckels K. H., Lai C. J. (2009). Passage of dengue virus type 4 vaccine candidates in fetal rhesus lung cells selects heparin-sensitive variants that result in loss of infectivity and immunogenicity in rhesus macaques. J. Virol. 83, 10384–10394 10.1128/JVI.01083-09 - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

