Topical application of PPADS inhibits complement activation and choroidal neovascularization in a model of age-related macular degeneration
- PMID: 24130789
- PMCID: PMC3793916
- DOI: 10.1371/journal.pone.0076766
Topical application of PPADS inhibits complement activation and choroidal neovascularization in a model of age-related macular degeneration
Abstract
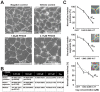
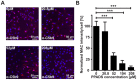
Age-related macular degeneration (AMD) is the most common cause of blindness among the elderly. AMD patients have elevated levels of membrane attack complex (MAC) in their choroidal blood vessels and retinal pigment epithelium (RPE). MAC forms pores in cell membranes. Low levels of MAC result in an elevation of cytokine release such as vascular endothelial growth factor (VEGF) that promotes the formation of choroidal neovascularization (CNV). High levels of MAC result in cell lysis and RPE degeneration is a hallmark of advanced AMD. The current standard of care for CNV associated with wet AMD is intravitreal injection of anti-VEGF molecules every 4 to 12 weeks. Such injections have significant side effects. Recently, it has been found that membrane pore-forming proteins such as α-haemolysin can mediate their toxic effects through auto- and paracrine signaling and that complement-induced lysis is amplified through ATP release followed by P2X receptor activation. We hypothesized that attenuation of P2X receptor activation may lead to a reduction in MAC deposition and consequent formation of CNV. Hence, in this study we investigated topical application of the purinergic P2X antagonist Pyridoxalphosphate-6-azophenyl-2',4'-disulphonic acid (PPADS) as a potential treatment for AMD. We found that 4.17 µM PPADS inhibited formation of HUVEC master junctions and master segments by 74.7%. In a human complement mediated cell lysis assay, 104 µM PPADS enabled almost complete protection of Hepa1c1c7 cells from 1% normal human serum mediated cell lysis. Daily topical application of 4.17 mM PPADS for 3 days attenuated the progression of laser induced CNV in mice by 41.8% and attenuated the deposition of MAC at the site of the laser injury by 19.7%. Our data have implications for the future treatment of AMD and potentially other ocular disorders involving CNV such as angioid streaks, choroidal rupture and high myopia.
Conflict of interest statement
Figures



References
-
- Lim LS, Mitchell P, Seddon JM, Holz FG, Wong TY (2012) Age-related macular degeneration. Lancet 379: 1728–1738. - PubMed
-
- Hageman GS, Luthert PJ, Victor Chong NH, Johnson LV, Anderson DH, et al. (2001) An integrated hypothesis that considers drusen as biomarkers of immune-mediated processes at the RPE-Bruch's membrane interface in aging and age-related macular degeneration. Prog Retin Eye Res 20: 705–732. - PubMed
-
- de Jong PT (2006) Age-related macular degeneration. N Engl J Med 355: 1474–1485. - PubMed
-
- Funk M, Karl D, Georgopoulos M, Benesch T, Sacu S, et al. (2009) Neovascular age-related macular degeneration: intraocular cytokines and growth factors and the influence of therapy with ranibizumab. Ophthalmology 116: 2393–2399. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

