Geometric and electronic structure of the Mn(IV)Fe(III) cofactor in class Ic ribonucleotide reductase: correlation to the class Ia binuclear non-heme iron enzyme
- PMID: 24131208
- PMCID: PMC3882272
- DOI: 10.1021/ja409510d
Geometric and electronic structure of the Mn(IV)Fe(III) cofactor in class Ic ribonucleotide reductase: correlation to the class Ia binuclear non-heme iron enzyme
Abstract
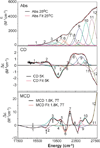
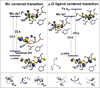
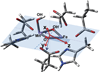
The class Ic ribonucleotide reductase (RNR) from Chlamydia trachomatis (Ct) utilizes a Mn/Fe heterobinuclear cofactor, rather than the Fe/Fe cofactor found in the β (R2) subunit of the class Ia enzymes, to react with O2. This reaction produces a stable Mn(IV)Fe(III) cofactor that initiates a radical, which transfers to the adjacent α (R1) subunit and reacts with the substrate. We have studied the Mn(IV)Fe(III) cofactor using nuclear resonance vibrational spectroscopy (NRVS) and absorption (Abs)/circular dichroism (CD)/magnetic CD (MCD)/variable temperature, variable field (VTVH) MCD spectroscopies to obtain detailed insight into its geometric/electronic structure and to correlate structure with reactivity; NRVS focuses on the Fe(III), whereas MCD reflects the spin-allowed transitions mostly on the Mn(IV). We have evaluated 18 systematically varied structures. Comparison of the simulated NRVS spectra to the experimental data shows that the cofactor has one carboxylate bridge, with Mn(IV) at the site proximal to Phe127. Abs/CD/MCD/VTVH MCD data exhibit 12 transitions that are assigned as d-d and oxo and OH(-) to metal charge-transfer (CT) transitions. Assignments are based on MCD/Abs intensity ratios, transition energies, polarizations, and derivative-shaped pseudo-A term CT transitions. Correlating these results with TD-DFT calculations defines the Mn(IV)Fe(III) cofactor as having a μ-oxo, μ-hydroxo core and a terminal hydroxo ligand on the Mn(IV). From DFT calculations, the Mn(IV) at site 1 is necessary to tune the redox potential to a value similar to that of the tyrosine radical in class Ia RNR, and the OH(-) terminal ligand on this Mn(IV) provides a high proton affinity that could gate radical translocation to the α (R1) subunit.
Figures







Similar articles
-
Two distinct mechanisms of inactivation of the class Ic ribonucleotide reductase from Chlamydia trachomatis by hydroxyurea: implications for the protein gating of intersubunit electron transfer.Biochemistry. 2010 Jun 29;49(25):5340-9. doi: 10.1021/bi100037b. Biochemistry. 2010. PMID: 20462199 Free PMC article.
-
Structural analysis of the Mn(IV)/Fe(III) cofactor of Chlamydia trachomatis ribonucleotide reductase by extended X-ray absorption fine structure spectroscopy and density functional theory calculations.J Am Chem Soc. 2008 Nov 12;130(45):15022-7. doi: 10.1021/ja804365e. Epub 2008 Oct 21. J Am Chem Soc. 2008. PMID: 18937466 Free PMC article.
-
High-valent [MnFe] and [FeFe] cofactors in ribonucleotide reductases.Biochim Biophys Acta. 2012 Mar;1817(3):430-44. doi: 10.1016/j.bbabio.2011.12.008. Epub 2011 Dec 23. Biochim Biophys Acta. 2012. PMID: 22222354
-
Formation and function of the Manganese(IV)/Iron(III) cofactor in Chlamydia trachomatis ribonucleotide reductase.Biochemistry. 2008 Dec 30;47(52):13736-44. doi: 10.1021/bi8017625. Biochemistry. 2008. PMID: 19061340 Free PMC article. Review.
-
The manganese(IV)/iron(III) cofactor of Chlamydia trachomatis ribonucleotide reductase: structure, assembly, radical initiation, and evolution.Curr Opin Struct Biol. 2008 Dec;18(6):650-7. doi: 10.1016/j.sbi.2008.11.007. Epub 2008 Nov 27. Curr Opin Struct Biol. 2008. PMID: 19046875 Review.
Cited by
-
Evidence for a Di-μ-oxo Diamond Core in the Mn(IV)/Fe(IV) Activation Intermediate of Ribonucleotide Reductase from Chlamydia trachomatis.J Am Chem Soc. 2017 Feb 8;139(5):1950-1957. doi: 10.1021/jacs.6b11563. Epub 2017 Jan 27. J Am Chem Soc. 2017. PMID: 28075562 Free PMC article.
-
Involvement of high-valent manganese-oxo intermediates in oxidation reactions: realisation in nature, nano and molecular systems.Nano Converg. 2018;5(1):18. doi: 10.1186/s40580-018-0150-5. Epub 2018 Jul 4. Nano Converg. 2018. PMID: 30101051 Free PMC article. Review.
-
Influence of the ligand-field on EPR parameters of cis- and trans-isomers in MoV systems relevant to molybdenum enzymes: Experimental and density functional theory study.J Inorg Biochem. 2023 Aug;245:112228. doi: 10.1016/j.jinorgbio.2023.112228. Epub 2023 Apr 24. J Inorg Biochem. 2023. PMID: 37149488 Free PMC article.
-
Magnetic circular dichroism and computational study of mononuclear and dinuclear iron(IV) complexes.Chem Sci. 2015 May 1;6(5):2909-2921. doi: 10.1039/C4SC03268C. Epub 2015 Feb 26. Chem Sci. 2015. PMID: 26417426 Free PMC article.
-
Stepwise assembly of heterobimetallic complexes: synthesis, structure, and physical properties.Dalton Trans. 2021 Jun 15;50(23):8111-8119. doi: 10.1039/d1dt01021b. Dalton Trans. 2021. PMID: 34019606 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

