Increased frequency of intestinal CD4+ T cells reactive with mycobacteria in patients with Crohn's disease
- PMID: 24131402
- PMCID: PMC3821379
- DOI: 10.3109/00365521.2013.837952
Increased frequency of intestinal CD4+ T cells reactive with mycobacteria in patients with Crohn's disease
Abstract
Objective: The aim of this study was to assess the frequency of mycobacteria and Escherichia coli reactive T cells in intestinal biopsies from patients with Crohn's disease (CD) and ulcerative colitis (UC).
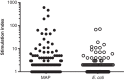
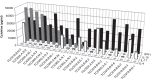
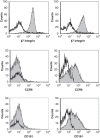
Materials and methods: The biopsies were obtained by colonoscopy from adult patients with active CD (n = 5) and active UC (n = 4). The number of CD4+ T cell clones expanded and screened from each patient varied from 383 to 3972 giving a total of 16639 individual clones. The T cell clones were tested for responses to Mycobacterium avium subspecies paratuberculosis (MAP) and E. coli. The cytokine profile of 42 individual T cell clones from four CD patients was assessed.
Results: The frequency of mycobacteria reactive T cell clones in CD patients ranged from 0.17 to 1.63% and was higher (p = 0.038) than the frequency of E. coli reactive T cells ranging from 0 to 0.18%. No or very low numbers of mycobacteria reactive clones were detected in three UC patients while the fourth UC patient had a frequency similar to what was observed in CD patients. The frequencies of E. coli reactive T cell clones in UC patients ranged from 0 to 0.52%. T cell clones (n = 42) from CD patients all produced IL-17 and/or IFN-γ. Several clones were also able to produce IL-10.
Conclusions: The high frequency of intestinal tissue resident T cells reactive to mycobacteria suggests that an adaptive immune response have taken place and argues that these bacteria may contribute to the chronic inflammation in CD.
Figures
References
-
- Zhang F, Liu H, Chen S, Low H, Sun L, Cui Y, et al. Identification of two new loci at IL23R and RAB32 that influence susceptibility to leprosy. Nat Genet. 2011;43:1247–51. - PubMed
-
- Khan KJ, Ullman TA, Ford AC, Abreu MT, Abadir A, Marshall JK, et al. Antibiotic therapy in inflammatory bowel disease: a systematic review and meta-analysis. Am J Gastroenterol. 2011;106:661–73. - PubMed
-
- Janowitz HD, Croen EC, Sachar DB. The role of the fecal stream in Crohn's disease: an historical and analytic review. Inflamm Bowel Dis. 1998;4:29–39. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
