Fetal adrenal capsular cells serve as progenitor cells for steroidogenic and stromal adrenocortical cell lineages in M. musculus
- PMID: 24131628
- PMCID: PMC3817941
- DOI: 10.1242/dev.092775
Fetal adrenal capsular cells serve as progenitor cells for steroidogenic and stromal adrenocortical cell lineages in M. musculus
Abstract
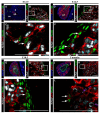
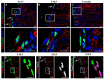
The lineage relationships of fetal adrenal cells and adrenal capsular cells to the differentiated adrenal cortex are not fully understood. Existing data support a role for each cell type as a progenitor for cells of the adult cortex. This report reveals that subsets of capsular cells are descendants of fetal adrenocortical cells that once expressed Nr5a1. These fetal adrenocortical cell descendants within the adrenal capsule express Gli1, a known marker of progenitors of steroidogenic adrenal cells. The capsule is also populated by cells that express Tcf21, a known inhibitor of Nr5a1 gene expression. We demonstrate that Tcf21-expressing cells give rise to Nr5a1-expressing cells but only before capsular formation. After the capsule has formed, capsular Tcf21-expressing cells give rise only to non-steroidogenic stromal adrenocortical cells, which also express collagen 1a1, desmin and platelet-derived growth factor (alpha polypeptide) but not Nr5a1. These observations integrate prior observations that define two separate origins of adult adrenocortical steroidogenic cells (fetal adrenal cortex and/or the adrenal capsule). Thus, these observations predict a unique temporal and/or spatial role of adult cortical cells that arise directly from either fetal cortical cells or from fetal cortex-derived capsular cells. Last, the data uncover the mechanism by which two populations of fetal cells (fetal cortex derived Gli1-expressing cells and mesenchymal Tcf21-expressing mesenchymal cells) participate in the establishment of the homeostatic capsular progenitor cell niche of the adult cortex.
Keywords: Adrenal gland; Homeostasis; Mouse; Progenitor cells.
Figures





References
-
- Ahn S., Joyner A. L. (2004). Dynamic changes in the response of cells to positive hedgehog signaling during mouse limb patterning. Cell 118, 505–516 - PubMed
-
- Bai C. B., Auerbach W., Lee J. S., Stephen D., Joyner A. L. (2002). Gli2, but not Gli1, is required for initial Shh signaling and ectopic activation of the Shh pathway. Development 129, 4753–4761 - PubMed
-
- Bingham N. C., Verma-Kurvari S., Parada L. F., Parker K. L. (2006). Development of a steroidogenic factor 1/Cre transgenic mouse line. Genesis 44, 419–424 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- G12 MD007601/MD/NIMHD NIH HHS/United States
- U01 HL100401/HL/NHLBI NIH HHS/United States
- T32 DK07245/DK/NIDDK NIH HHS/United States
- R01 DK062027/DK/NIDDK NIH HHS/United States
- T32 HL115505/HL/NHLBI NIH HHS/United States
- CA134606/CA/NCI NIH HHS/United States
- P30 GM103341/GM/NIGMS NIH HHS/United States
- P30 DK020572/DK/NIDDK NIH HHS/United States
- R01 CA134606/CA/NCI NIH HHS/United States
- HL074257/HL/NHLBI NIH HHS/United States
- HL100401/HL/NHLBI NIH HHS/United States
- R01 HL074257/HL/NHLBI NIH HHS/United States
- T32 DK007245/DK/NIDDK NIH HHS/United States
- T32 HD007048/HD/NICHD NIH HHS/United States
- DK062027/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

