Chronic joint disease caused by persistent Chikungunya virus infection is controlled by the adaptive immune response
- PMID: 24131709
- PMCID: PMC3838294
- DOI: 10.1128/JVI.02666-13
Chronic joint disease caused by persistent Chikungunya virus infection is controlled by the adaptive immune response
Abstract
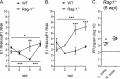
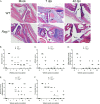
Chikungunya virus (CHIKV) is a reemerging mosquito-borne pathogen that causes incapacitating disease in humans characterized by intense joint pain that can persist for weeks, months, or even years. Although there is some evidence of persistent CHIKV infection in humans suffering from chronic rheumatologic disease symptoms, little is known about chronic disease pathogenesis, and no specific therapies exist for acute or chronic CHIKV disease. To investigate mechanisms of chronic CHIKV-induced disease, we utilized a mouse model and defined the duration of CHIKV infection in tissues and the associated histopathological changes. Although CHIKV RNA was readily detectable in a variety of tissues very early after infection, CHIKV RNA persisted specifically in joint-associated tissues for at least 16 weeks. Inoculation of Rag1(-/-) mice, which lack T and B cells, resulted in higher viral levels in a variety of tissues, suggesting that adaptive immunity controls the tissue specificity and persistence of CHIKV infection. The presence of CHIKV RNA in tissues of wild-type and Rag1(-/-) mice was associated with histopathological evidence of synovitis, arthritis, and tendonitis; thus, CHIKV-induced persistent arthritis is not mediated primarily by adaptive immune responses. Finally, we show that prophylactic administration of CHIKV-specific monoclonal antibodies prevented the establishment of CHIKV persistence, whereas therapeutic administration had tissue-specific efficacy. These findings suggest that chronic musculoskeletal tissue pathology is caused by persistent CHIKV infection and controlled by adaptive immune responses. Our results have significant implications for the development of strategies to mitigate the disease burden associated with CHIKV infection in humans.
Figures




References
-
- Suhrbier A, Jaffar-Bandjee MC, Gasque P. 2012. Arthritogenic alphaviruses—an overview. Nat. Rev. Rheumatol. 8:420–429 - PubMed
-
- Burt FJ, Rolph MS, Rulli NE, Mahalingam S, Heise MT. 2012. Chikungunya: a re-emerging virus. Lancet 379:662–671 - PubMed
-
- Powers AM, Logue CH. 2007. Changing patterns of Chikungunya virus: re-emergence of a zoonotic arbovirus. J. Gen. Virol. 88:2363–2377 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

