Elucidation of the block to herpes simplex virus egress in the absence of tegument protein UL16 reveals a novel interaction with VP22
- PMID: 24131716
- PMCID: PMC3911764
- DOI: 10.1128/JVI.02555-13
Elucidation of the block to herpes simplex virus egress in the absence of tegument protein UL16 reveals a novel interaction with VP22
Abstract
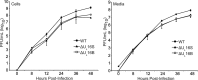
UL16 is a tegument protein of herpes simplex virus (HSV) that is conserved among all members of the Herpesviridae, but its function is poorly understood. Previous studies revealed that UL16 is associated with capsids in the cytoplasm and interacts with the membrane protein UL11, which suggested a "bridging" function during cytoplasmic envelopment, but this conjecture has not been tested. To gain further insight, cells infected with UL16-null mutants were examined by electron microscopy. No defects in the transport of capsids to cytoplasmic membranes were observed, but the wrapping of capsids with membranes was delayed. Moreover, clusters of cytoplasmic capsids were often observed, but only near membranes, where they were wrapped to produce multiple capsids within a single envelope. Normal virion production was restored when UL16 was expressed either by complementing cells or from a novel position in the HSV genome. When the composition of the UL16-null viruses was analyzed, a reduction in the packaging of glycoprotein E (gE) was observed, which was not surprising, since it has been reported that UL16 interacts with this glycoprotein. However, levels of the tegument protein VP22 were also dramatically reduced in virions, even though this gE-binding protein has been shown not to depend on its membrane partner for packaging. Cotransfection experiments revealed that UL16 and VP22 can interact in the absence of other viral proteins. These results extend the UL16 interaction network beyond its previously identified binding partners to include VP22 and provide evidence that UL16 plays an important function at the membrane during virion production.
Figures









References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

