Prebiotic and probiotic supplementation prevents rhinovirus infections in preterm infants: a randomized, placebo-controlled trial
- PMID: 24131826
- PMCID: PMC7112326
- DOI: 10.1016/j.jaci.2013.08.020
Prebiotic and probiotic supplementation prevents rhinovirus infections in preterm infants: a randomized, placebo-controlled trial
Abstract
Background: Simple and safe strategies for the prevention of viral respiratory tract infections (RTIs) are needed.
Objective: We hypothesized that early prebiotic or probiotic supplementation would reduce the risk of virus-associated RTIs during the first year of life in a cohort of preterm infants.
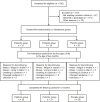
Methods: In this randomized, double-blind, placebo-controlled trial (ClinicalTrials.gov no. NCT00167700), 94 preterm infants (gestational age, ≥32 + 0 and ≤36 + 6 weeks; birth weight, >1500 g) treated at Turku University Hospital, Turku, Finland, were allocated to receive oral prebiotics (galacto-oligosaccharide and polydextrose mixture, 1:1), a probiotic (Lactobacillus rhamnosus GG, ATCC 53103), or placebo (microcrystalline cellulose) between days 3 and 60 of life. The primary outcome was the incidence of clinically defined virus-associated RTI episodes confirmed from nasal swabs by using nucleic acid testing. Secondary outcomes were the severity and duration of RTIs.
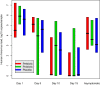
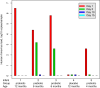
Results: A significantly lower incidence of RTIs was detected in infants receiving prebiotics (rate ratio [RR], 0.24; 95% CI, 0.12-0.49; P < .001) or probiotics (RR, 0.50; 95% CI, 0.28-0.90; P = .022) compared with those receiving placebo. Also, the incidence of rhinovirus-induced episodes, which comprised 80% of all RTI episodes, was found to be significantly lower in the prebiotic (RR, 0.31; 95% CI, 0.14-0.66; P = .003) and probiotic (RR, 0.49; 95% CI, 0.24-1.00; P = .051) groups compared with the placebo group. No differences emerged among the study groups in rhinovirus RNA load during infections, duration of rhinovirus RNA shedding, duration or severity of rhinovirus infections, or occurrence of rhinovirus RNA in asymptomatic infants.
Conclusions: Gut microbiota modification with specific prebiotics and probiotics might offer a novel and cost-effective means to reduce the risk of rhinovirus infections.
Keywords: Galacto-oligosaccharide; Lactobacillus rhamnosus GG; RR; RSV; RTI; Rate ratio; Respiratory syncytial virus; Respiratory tract infection; gut microbiota; polydextrose; prebiotic; preterm infant; probiotic; respiratory tract infections; rhinovirus.
Copyright © 2013 American Academy of Allergy, Asthma & Immunology. Published by Mosby, Inc. All rights reserved.
Figures
References
-
- Food and Agriculture Organization of the United Nations, World Health Organization. Report of a joint FAO/WHO expert consultation on evaluation of health and nutritional properties of probiotics in food including powder milk with live lactic acid bacteria. Available at: www.who.int/entity/foodsafety/publications/fs_management/en/probiotics.pdf. Accessed November 2009.
-
- Pineiro M., Asp N.G., Reid G., Macfarlane S., Morelli L., Brunser O. FAO Technical meeting on prebiotics. J Clin Gastroenterol. 2008;42(Suppl 3 Pt 2):S156–S159. - PubMed
-
- Vouloumanou E.K., Makris G.C., Karageorgopoulos D.E., Falagas M.E. Probiotics for the prevention of respiratory tract infections: a systematic review. Int J Antimicrob Agents. 2009;34:197. - PubMed
-
- Weichert S., Schroten H., Adam R. The role of prebiotics and probiotics in prevention and treatment of childhood infectious diseases. Pediatr Infect Dis J. 2012;31:859–862. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

