Adeno-associated virus serotypes 9 and rh10 mediate strong neuronal transduction of the dog brain
- PMID: 24131981
- PMCID: PMC3881028
- DOI: 10.1038/gt.2013.54
Adeno-associated virus serotypes 9 and rh10 mediate strong neuronal transduction of the dog brain
Abstract
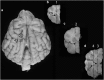
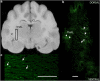
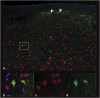
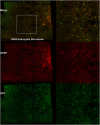
Canine models have many advantages for evaluating therapy of human central nervous system (CNS) diseases. In contrast to nonhuman primate models, naturally occurring canine CNS diseases are common. In contrast to murine models, the dog's lifespan is long, its brain is large and the diseases affecting it commonly have the same molecular, pathological and clinical phenotype as the human diseases. We compared the ability of four intracerebrally injected adeno-associated virus vector (AAV) serotypes to transduce the dog brain with green fluorescent protein as the first step in using these vectors to evaluate both delivery and efficacy in naturally occurring canine homologs of human diseases. Quantitative measures of transduction, maximum diameter and area, identified both AAV2/9 and AAV2/rh10 as significantly more efficient than either AAV2/1 or AAV2/5 at transducing cerebral cortex, caudate nucleus, thalamus and internal capsule. Fluorescence co-labeling with cell-type-specific antibodies demonstrated that AAV2/9 and AAV2/rh10 were capable of primarily transducing neurons, although glial transduction was also identified and found to be more efficient with the AAV2/9 vector. These data are a prerequisite to evaluating the efficacy of recombinant AAV vectors carrying disease-modifying transgenes to treat naturally occurring canine models in preclinical studies of human CNS disease therapy.
Figures


Similar articles
-
Transduction of the central nervous system after intracerebroventricular injection of adeno-associated viral vectors in neonatal and juvenile mice.Hum Gene Ther Methods. 2013 Aug;24(4):205-13. doi: 10.1089/hgtb.2013.076. Epub 2013 Aug 3. Hum Gene Ther Methods. 2013. PMID: 23808551 Free PMC article.
-
Adeno-associated virus (AAV) serotypes 2, 4 and 5 display similar transduction profiles and penetrate solid tumor tissue in models of human glioma.J Gene Med. 2006 Sep;8(9):1131-40. doi: 10.1002/jgm.939. J Gene Med. 2006. PMID: 16810631
-
Adeno-associated viral serotypes differentially transduce inhibitory neurons within the rat amygdala.Brain Res. 2017 Oct 1;1672:148-162. doi: 10.1016/j.brainres.2017.07.023. Epub 2017 Jul 29. Brain Res. 2017. PMID: 28764932
-
Cellular selectivity of AAV serotypes for gene delivery in neurons and astrocytes by neonatal intracerebroventricular injection.PLoS One. 2017 Dec 15;12(12):e0188830. doi: 10.1371/journal.pone.0188830. eCollection 2017. PLoS One. 2017. PMID: 29244806 Free PMC article.
-
Serotype-independent method of recombinant adeno-associated virus (AAV) vector production and purification.J Nippon Med Sch. 2012;79(6):394-402. doi: 10.1272/jnms.79.394. J Nippon Med Sch. 2012. PMID: 23291836 Review.
Cited by
-
AAVrh10 Gene Therapy Ameliorates Central and Peripheral Nervous System Disease in Canine Globoid Cell Leukodystrophy (Krabbe Disease).Hum Gene Ther. 2018 Jul;29(7):785-801. doi: 10.1089/hum.2017.151. Epub 2018 Mar 14. Hum Gene Ther. 2018. PMID: 29316812 Free PMC article.
-
Recent progress and considerations for AAV gene therapies targeting the central nervous system.J Neurodev Disord. 2018 May 18;10(1):16. doi: 10.1186/s11689-018-9234-0. J Neurodev Disord. 2018. PMID: 29776328 Free PMC article. Review.
-
Clustered Regularly Interspaced Short Palindromic Repeats-Based Genome Surgery for the Treatment of Autosomal Dominant Retinitis Pigmentosa.Ophthalmology. 2018 Sep;125(9):1421-1430. doi: 10.1016/j.ophtha.2018.04.001. Epub 2018 May 11. Ophthalmology. 2018. PMID: 29759820 Free PMC article.
-
Efficient gene delivery to photoreceptors using AAV2/rh10 and rescue of the Rho(-/-) mouse.Mol Ther Methods Clin Dev. 2015 May 6;2:15016. doi: 10.1038/mtm.2015.16. eCollection 2015. Mol Ther Methods Clin Dev. 2015. PMID: 26029727 Free PMC article.
-
Efficient central nervous system AAVrh10-mediated intrathecal gene transfer in adult and neonate rats.Gene Ther. 2015 Apr;22(4):316-24. doi: 10.1038/gt.2014.121. Epub 2015 Jan 15. Gene Ther. 2015. PMID: 25588740
References
-
- Haskins M, Giger U.Lysosomal storage diseasesIn: Kaneko JJ, Harvey JW, Bruss ML (eds.)Clinical Biochemistry of Domestic Animals6th edn.Elsevier: Amsterdam; 2008731–750.
-
- Zeng R, Farias FH, Johnson GS, McKay SD, Schnabel RD, Decker JE, et al. A truncated retrotransposon disrupts the GRM1 coding sequence in Coton de Tulear dogs with Bandera's neonatal ataxia. J Vet Intern Med. 2011;25:267–272. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

