The microbiome and cancer
- PMID: 24132111
- PMCID: PMC3986062
- DOI: 10.1038/nrc3610
The microbiome and cancer
Abstract
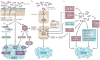
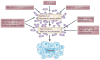
Microbiota and host form a complex 'super-organism' in which symbiotic relationships confer benefits to the host in many key aspects of life. However, defects in the regulatory circuits of the host that control bacterial sensing and homeostasis, or alterations of the microbiome, through environmental changes (infection, diet or lifestyle), may disturb this symbiotic relationship and promote disease. Increasing evidence indicates a key role for the bacterial microbiota in carcinogenesis. In this Opinion article, we discuss links between the bacterial microbiota and cancer, with a particular focus on immune responses, dysbiosis, genotoxicity, metabolism and strategies to target the microbiome for cancer prevention.
Conflict of interest statement
The authors declare no competing financial interests.
Figures

References
-
- Koch R. Tenth International Congress of Medicine 1. August Hirschwald; Berlin: 1891.
-
- Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet. 2001;357:539–545. - PubMed
-
- Trinchieri G. Cancer and inflammation: an old intuition with rapidly evolving new concepts. Annu Rev Immunol. 2012;30:677–706. - PubMed
-
- Virchow R. In: Die krankhaften Geschwülste. Virchow R, editor. Verlag von August von Hirschwald; Berlin: 1863. pp. 57–101.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

