SHANK3 and IGF1 restore synaptic deficits in neurons from 22q13 deletion syndrome patients
- PMID: 24132240
- PMCID: PMC5559273
- DOI: 10.1038/nature12618
SHANK3 and IGF1 restore synaptic deficits in neurons from 22q13 deletion syndrome patients
Abstract
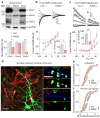
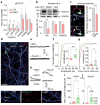
Phelan-McDermid syndrome (PMDS) is a complex neurodevelopmental disorder characterized by global developmental delay, severely impaired speech, intellectual disability, and an increased risk of autism spectrum disorders (ASDs). PMDS is caused by heterozygous deletions of chromosome 22q13.3. Among the genes in the deleted region is SHANK3, which encodes a protein in the postsynaptic density (PSD). Rare mutations in SHANK3 have been associated with idiopathic ASDs, non-syndromic intellectual disability, and schizophrenia. Although SHANK3 is considered to be the most likely candidate gene for the neurological abnormalities in PMDS patients, the cellular and molecular phenotypes associated with this syndrome in human neurons are unknown. We generated induced pluripotent stem (iPS) cells from individuals with PMDS and autism and used them to produce functional neurons. We show that PMDS neurons have reduced SHANK3 expression and major defects in excitatory, but not inhibitory, synaptic transmission. Excitatory synaptic transmission in PMDS neurons can be corrected by restoring SHANK3 expression or by treating neurons with insulin-like growth factor 1 (IGF1). IGF1 treatment promotes formation of mature excitatory synapses that lack SHANK3 but contain PSD95 and N-methyl-D-aspartate (NMDA) receptors with fast deactivation kinetics. Our findings provide direct evidence for a disruption in the ratio of cellular excitation and inhibition in PMDS neurons, and point to a molecular pathway that can be recruited to restore it.
Figures



References
-
- Boeckers TM, Bockmann J, Kreutz MR, Gundelfinger ED. ProSAP/Shank proteins - a family of higher order organizing molecules of the postsynaptic density with an emerging role in human neurological disease. J Neurochem. 2002;81:903–910. - PubMed
-
- Sheng M, Kim E. The Shank family of scaffold proteins. J Cell Sci. 2000;113(Pt 11):1851–1856. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

