Control and modulation of fluid flow in the rat kidney
- PMID: 24132579
- PMCID: PMC3870166
- DOI: 10.1007/s11538-013-9907-5
Control and modulation of fluid flow in the rat kidney
Abstract
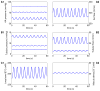
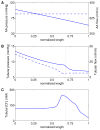
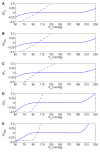
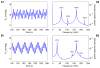
We have developed a mathematical model of the rat's renal hemodynamics in the nephron level, and used that model to study flow control and signal transduction in the rat kidney. The model represents an afferent arteriole, glomerular filtration, and a segment of a short-loop nephron. The model afferent arteriole is myogenically active and represents smooth muscle membrane potential and electrical coupling. The myogenic mechanism is based on the assumption that the activity of nonselective cation channels is shifted by changes in transmural pressure, such that elevation in pressure induces vasoconstriction, which increases resistance to blood flow. From the afferent arteriole's fluid delivery output, glomerular filtration rate is computed, based on conservation of plasma and plasma protein. Chloride concentration is then computed along the renal tubule based on solute conservation that represents water reabsorption along the proximal tubule and the water-permeable segment of the descending limb, and chloride fluxes driven by passive diffusion and active transport. The model's autoregulatory response is predicted to maintain stable renal blood flow within a physiologic range of blood pressure values. Power spectra associated with time series predicted by the model reveal a prominent fundamental peak at ∼165 mHz arising from the afferent arteriole's spontaneous vasomotion. Periodic external forcings interact with vasomotion to introduce heterodynes into the power spectra, significantly increasing their complexity.
Figures




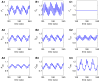
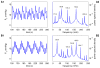
References
-
- Chon KH, Raghavan R, Chen Y-M, Marsh DJ, Yip K-P. Interactions of TGF-dependent and myogenic oscillations in tubular pressure. Am J Physiol|Renal Physiol. 2005;288:F298–F307. - PubMed
-
- Cupples WA, Braam B. Assessment of renal autoregulation. American Journal of Physiology-Renal Physiology. 2007;292(4):F1105–F1123. - PubMed
-
- Deen WM, Robertson CR, Brenner BM. A model of glomerular ultrafiltration in the rat. Am J Physiol. 1972;223(5):1178–1183. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

