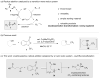
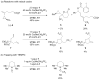
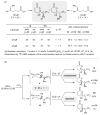
Enantioselective functionalization of radical intermediates in redox catalysis: copper-catalyzed asymmetric oxytrifluoromethylation of alkenes
- PMID: 24133010
- PMCID: PMC3880552
- DOI: 10.1002/anie.201307790
Enantioselective functionalization of radical intermediates in redox catalysis: copper-catalyzed asymmetric oxytrifluoromethylation of alkenes
Abstract
A method for the efficient enantioselective oxytrifluoromethylation of alkenes has been developed using a copper catalyst system. Mechanistic studies are consistent with a metal-catalyzed redox radical addition mechanism, in which a C–O bond is formed via the copper-mediated enantioselective trapping of a prochiral alkyl radical intermediate derived from the initial trifluoromethyl radical addition.
Keywords: asymmetric catalysis; copper; enantioselectivity; radicals; synthetic methods.
Figures
References
-
-
Selected recent examples: Pathak TP, Gligorich KM, Welm BE, Sigman MS. J Am Chem Soc. 2010;132:7870.Melhado AD, Brenzovich WE, Lackner AD, Toste FD. J Am Chem Soc. 2010;132:8885.Zhang G, Cui L, Wang Y, Zhang L. J Am Chem Soc. 2010;132:1474.Liwosz TW, Chemler SR. J Am Chem Soc. 2012;134:2020.Sahoo B, Hopkinson MN, Glorius F. J Am Chem Soc. 2013;135:5505.Nguyen JD, Tucker JW, Konieczynska MD, Stephenson CRJ. J Am Chem Soc. 2011;133:4160.Liu G, Stahl SS. J Am Chem Soc. 2007;129:6328.Martínez C, Muñiz K. Angew Chem. 2012;124:7138.Angew Chem Int Ed. 2012;51:7031.Hopkins BA, Wolfe JP. Angew Chem. 2012;124:10024.Angew Chem Int Ed. 2012;51:9886.Alexanian EJ, Lee CB, Sorensen EJ. J Am Chem Soc. 2005;127:7690.Kalyani D, Sanford MS. J Am Chem Soc. 2008;130:2150.Rosewall CF, Sibbald PA, Liskin DV, Michael FE. J Am Chem Soc. 2009;131:9488.Kirchberg S, Froehlich R, Studer A. Angew Chem. 2010;122:7029.Angew Chem Int Ed. 2010;49:6877.Nicolai S, Piemontesi C, Waser J. Angew Chem. 2011;123:4776.Angew Chem Int Ed. 2011;50:4680.Zhu MK, Zhao JF, Loh TP. J Am Chem Soc. 2010;132:6284.Zhao B, Peng X, Zhu Y, Ramirez TA, Cornwall RG, Shi Y. J Am Chem Soc. 2011;133:20890.Matsuda N, Hirano K, Satoh T, Miura M. J Am Chem Soc. 2013;135:4934.
-
-
- Minisci F. Acc Chem Res. 1975;8:165.
- Wallentin CJ, Nguyen JD, Finkbeiner P, Stephenson CRJ. J Am Chem Soc. 2012;134:8875. - PubMed
-
- Murai S, Sugise R, Sonoda N. Angew Chem. 1981;93:481.
- Angew Chem Int Ed. 1981;20:475.
- Kameyama M, Kamigata N, Kobayashi M. J Org Chem. 1987;52:3312.
- Iizuka Y, Li Z, Satoh K, Kamigaito M, Okamoto Y, Ito J, Nishiyama H. Eur J Org Chem. 2007:782.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources