Regulation of NKCC2 activity by inhibitory SPAK isoforms: KS-SPAK is a more potent inhibitor than SPAK2
- PMID: 24133122
- PMCID: PMC3882447
- DOI: 10.1152/ajprenal.00211.2013
Regulation of NKCC2 activity by inhibitory SPAK isoforms: KS-SPAK is a more potent inhibitor than SPAK2
Abstract
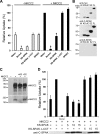
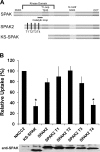
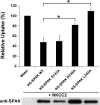
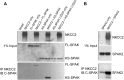
The cation cotransporters Na(+)-K(+)-2Cl(-) cotransporter 1 and 2 (NKCC1 and NKCC2) and Na(+)-Cl cotransporter (NCC) are phosphorylated and activated by the kinases Ste20-related proline alanine-rich kinase (SPAK) and oxidative stress-responsive kinase (OSR1), and their targeted disruption in mice causes phenotypes resembling the human disorders Bartter syndrome and Gitelman syndrome, reflecting reduced NKCC2 and NCC activity, respectively. We previously cloned a kinase-inactive kidney-specific SPAK isoform, kidney-specific (KS)-SPAK, which lacks the majority of the kinase domain present in full-length SPAK. Another putative inactive SPAK isoform, SPAK2, which only lacks the initial portion of the kinase domain, is also highly expressed in kidney. The functional relevance of inactive SPAK isoforms is unclear. Here, we tested whether KS-SPAK and SPAK2 differentially affect cation cotransporter activity. While KS-SPAK and SPAK2 both strongly inhibited NKCC1 activity, SPAK2 was a much weaker inhibitor of NKCC2 activity. Removal of the catalytic loop from SPAK2 resulted in an inhibitory effect on NKCC2 similar to that of KS-SPAK. Full-length SPAK is phosphorylated and activated by members of the with-no-lysine[K] (WNK) kinase family. Mutation of a WNK phosphorylation in KS-SPAK did not alter its ability to inhibit NKCC2 activity. In contrast, we found that residues involved in KS-SPAK interactions with cation cotransporters are required for it to inhibit cotransporter activity. Finally, both KS-SPAK and SPAK2 associated with NKCC2, as demonstrated by coimmunoprecipitation. Together, these data identify the structural basis for the differential effects of KS-SPAK and SPAK2 on cation cotransporter activity that may be physiologically important.
Keywords: cation cotransporter; hypertension; kinase.
Figures

Comment in
-
Regulation of NKCC2 activity by SPAK truncated isoforms.Am J Physiol Renal Physiol. 2014 Jan 1;306(1):F49-50. doi: 10.1152/ajprenal.00559.2013. Epub 2013 Oct 30. Am J Physiol Renal Physiol. 2014. PMID: 24173353 No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

