Microvascular COX-2/mPGES-1/EP-4 axis in human abdominal aortic aneurysm
- PMID: 24133193
- PMCID: PMC3826696
- DOI: 10.1194/jlr.M042481
Microvascular COX-2/mPGES-1/EP-4 axis in human abdominal aortic aneurysm
Abstract
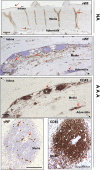
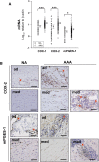
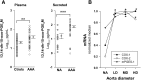
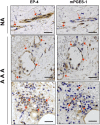
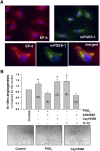
We investigated the prostaglandin (PG)E2 pathway in human abdominal aortic aneurysm (AAA) and its relationship with hypervascularization. We analyzed samples from patients undergoing AAA repair in comparison with those from healthy multiorgan donors. Patients were stratified according to maximum aortic diameter: low diameter (LD) (<55 mm), moderate diameter (MD) (55-69.9 mm), and high diameter (HD) (≥70 mm). AAA was characterized by abundant microvessels in the media and adventitia with perivascular infiltration of CD45-positive cells. Like endothelial cell markers, cyclooxygenase (COX)-2 and the microsomal isoform of prostaglandin E synthase (mPGES-1) transcripts were increased in AAA (4.4- and 1.4-fold, respectively). Both enzymes were localized in vascular cells and leukocytes, with maximal expression in the LD group, whereas leukocyte markers display a maximum in the MD group, suggesting that the upregulation of COX-2/mPGES-1 precedes maximal leukocyte infiltration. Plasma and in vitro tissue secreted levels of PGE2 metabolites were higher in AAA than in controls (plasma-controls, 19.9 ± 2.2; plasma-AAA, 38.8 ± 5.5 pg/ml; secretion-normal aorta, 16.5 ± 6.4; secretion-AAA, 72.9 ± 6.4 pg/mg; mean ± SEM). E-prostanoid receptor (EP)-2 and EP-4 were overexpressed in AAA, EP-4 being the only EP substantially expressed and colocalized with mPGES-1 in the microvasculature. Additionally, EP-4 mediated PGE2-induced angiogenesis in vitro. We provide new data concerning mPGES-1 expression in human AAA. Our findings suggest the potential relevance of the COX-2/mPGES-1/EP-4 axis in the AAA-associated hypervascularization.
Keywords: E prostanoid receptor; angiogenesis; cyclooxygenase; cyclooxygenase pathway; microsomal isoform of prostaglandin E synthase; prostaglandin.
Figures


References
-
- Wahlgren C. M., Larsson E., Magnusson P. K. E., Hultgren R., Swadenborg J. 2010. Genetic and enviromental contributions to abdominal aortic aneurysm development in twin population. J. Vasc. Surg. 51: 3–7 - PubMed
-
- Choke E., Cockerill G., Wilson W. R., Sayed S., Dawson J., Loftus I., Thompson M. M. 2005. A review of biological factors implicated in abdominal aortic aneurysm rupture. Eur. J. Vasc. Endovasc. Surg. 30: 227–244 - PubMed
-
- Paik D. C., Fu C., Bhattacharya J., Tilson M. D. 2004. Ongoing angiogenesis in blood vessels of the abdominal aortic aneurysm. Exp. Mol. Med. 36: 524–533 - PubMed
-
- Choke E., Thompson M. M., Dawson J., Wilson W. R., Sayed S., Loftus I. M., Cockerill G. W. 2006. Abdominal aortic aneurysm rupture is associated with increased medial neovascularization and overexpression of proangiogenic cytokines. Arterioscler. Thromb. Vasc. Biol. 26: 2077–2082 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

