Identification of a dithiol-disulfide switch in collapsin response mediator protein 2 (CRMP2) that is toggled in a model of neuronal differentiation
- PMID: 24133216
- PMCID: PMC3853263
- DOI: 10.1074/jbc.M113.521443
Identification of a dithiol-disulfide switch in collapsin response mediator protein 2 (CRMP2) that is toggled in a model of neuronal differentiation
Abstract
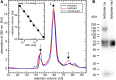
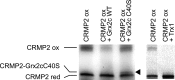
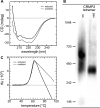
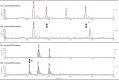
Vertebrate-specific glutaredoxin 2 (Grx2) is expressed in at least two isoforms, mitochondrial Grx2a and cytosolic Grx2c. We have previously shown that cytosolic Grx2 is essential for embryonic development of the brain. In particular, we identified collapsin response mediator protein 2 (CRMP2/DPYSL2), a mediator of the semaphorin-plexin signaling pathway, as redox-regulated target of Grx2c and demonstrated that this regulation is required for normal axonal outgrowth. In this study, we demonstrate the molecular mechanism of this regulation, a specific and reversible intermolecular Cys-504-Cys-504 dithiol-disulfide switch in homotetrameric CRMP2. This switch determines two conformations of the quaternary CRMP2 complex that controls axonal outgrowth and thus neuronal development.
Keywords: CRMP2; Disulfide; Glutaredoxin; Neurodifferentiation; Neuronal Differentiation; Redox Regulation; Redox Signaling; Redox Switch; Thiol.
Figures

References
-
- Holmgren A. (1979) Glutathione-dependent synthesis of deoxyribonucleotides. Purification and characterization of glutaredoxin from Escherichia coli. J. Biol. Chem. 254, 3664–3671 - PubMed
-
- Gravina S. A., Mieyal J. J. (1993) Thioltransferase is a specific glutathionyl mixed disulfide oxidoreductase. Biochemistry 32, 3368–3376 - PubMed
-
- Lillig C. H., Berndt C., Holmgren A. (2008) Glutaredoxin systems. Biochim. Biophys. Acta 1780, 1304–1317 - PubMed
-
- Lillig C. H., Berndt C. (2013) Glutaredoxins in thiol/disulfide exchange. Antioxid. Redox Signal. 18, 1654–1665 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

