The role of extracellular vesicles in phenotypic cancer transformation
- PMID: 24133383
- PMCID: PMC3794874
- DOI: 10.2478/raon-2013-0037
The role of extracellular vesicles in phenotypic cancer transformation
Abstract
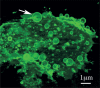
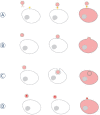
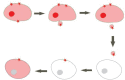
Background: Cancer has traditionally been considered as a disease resulting from gene mutations. New findings in biology are challenging gene-centered explanations of cancer progression and redirecting them to the non-genetic origins of tumorigenicity. It has become clear that intercellular communication plays a crucial role in cancer progression. Among the most intriguing ways of intercellular communication is that via extracellular vesicles (EVs). EVs are membrane structures released from various types of cells. After separation from the mother membrane, EVs become mobile and may travel from the extracellular space to blood and other body fluids.
Conclusions: Recently it has been shown that tumour cells are particularly prone to vesiculation and that tumour-derived EVs can carry proteins, lipids and nucleic acids causative of cancer progression. The uptake of tumour-derived EVs by noncancerous cells can change their normal phenotype to cancerous. The suppression of vesiculation could slow down tumour growth and the spread of metastases. The purpose of this review is to highlight examples of EV-mediated cancer phenotypic transformation in the light of possible therapeutic applications.
Keywords: cancer progression; exosomes; extracellular vesicles; microvesicles.
Figures


Similar articles
-
Extracellular vesicle communication pathways as regulatory targets of oncogenic transformation.Semin Cell Dev Biol. 2017 Jul;67:11-22. doi: 10.1016/j.semcdb.2017.01.003. Epub 2017 Jan 8. Semin Cell Dev Biol. 2017. PMID: 28077296 Review.
-
The Yin and Yang of tumour-derived extracellular vesicles in tumour immunity.J Biochem. 2021 Mar 5;169(2):155-161. doi: 10.1093/jb/mvaa132. J Biochem. 2021. PMID: 33226400
-
Tumor-derived extracellular vesicles: molecular parcels that enable regulation of the immune response in cancer.J Cell Sci. 2019 Oct 15;132(20):jcs235085. doi: 10.1242/jcs.235085. J Cell Sci. 2019. PMID: 31615844 Free PMC article. Review.
-
Routes and mechanisms of extracellular vesicle uptake.J Extracell Vesicles. 2014 Aug 4;3. doi: 10.3402/jev.v3.24641. eCollection 2014. J Extracell Vesicles. 2014. PMID: 25143819 Free PMC article. Review.
-
Extracellular Vesicles: New Endogenous Shuttles for miRNAs in Cancer Diagnosis and Therapy?Int J Mol Sci. 2020 Sep 4;21(18):6486. doi: 10.3390/ijms21186486. Int J Mol Sci. 2020. PMID: 32899898 Free PMC article. Review.
Cited by
-
Cambinol, a novel inhibitor of neutral sphingomyelinase 2 shows neuroprotective properties.PLoS One. 2015 May 26;10(5):e0124481. doi: 10.1371/journal.pone.0124481. eCollection 2015. PLoS One. 2015. PMID: 26010541 Free PMC article.
-
Extracellular Vesicles Released by Colorectal Cancer Cell Lines Modulate Innate Immune Response in Zebrafish Model: The Possible Role of Human Endogenous Retroviruses.Int J Mol Sci. 2019 Jul 26;20(15):3669. doi: 10.3390/ijms20153669. Int J Mol Sci. 2019. PMID: 31357477 Free PMC article.
-
Navigating the Landscape of Tumor Extracellular Vesicle Heterogeneity.Int J Mol Sci. 2019 Mar 18;20(6):1349. doi: 10.3390/ijms20061349. Int J Mol Sci. 2019. PMID: 30889795 Free PMC article. Review.
-
Exosomes Derived from Breast Cancer Cells, Small Trojan Horses?J Mammary Gland Biol Neoplasia. 2014 Dec;19(3-4):303-13. doi: 10.1007/s10911-015-9332-5. Epub 2015 Jul 1. J Mammary Gland Biol Neoplasia. 2014. PMID: 26130410 Review.
-
Tumor-derived exosomes in the regulation of macrophage polarization.Inflamm Res. 2020 May;69(5):435-451. doi: 10.1007/s00011-020-01318-0. Epub 2020 Mar 11. Inflamm Res. 2020. PMID: 32162012 Review.
References
-
- Al-Nedawi K, Meehan B, Rak J. Microvesicles: messengers and mediators of tumor progression. Cell Cycle. 2009;8:2014–18. - PubMed
-
- Rak J. Microparticles in cancer. Semin Thromb Hemost. 2010;36:888–906. - PubMed
-
- Pap E. The role of microvesicles in malignancies. Adv Exp Med Biol. 2011;714:183–199. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
