Targeting cardiac β-adrenergic signaling via GRK2 inhibition for heart failure therapy
- PMID: 24133451
- PMCID: PMC3783981
- DOI: 10.3389/fphys.2013.00264
Targeting cardiac β-adrenergic signaling via GRK2 inhibition for heart failure therapy
Abstract
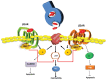
Cardiac cells, like those of the other tissues, undergo regulation through membrane-bound proteins known as G protein-coupled receptors (GPCRs). β-adrenergic receptors (βARs) are key GPCRs expressed on cardiomyocytes and their role is crucial in cardiac physiology since they regulate inotropic and chronotropic responses of the sympathetic nervous system (SNS). In compromised conditions such as heart failure (HF), chronic βAR hyperstimulation occurs via SNS activation resulting in receptor dysregulation and down-regulation and consequently there is a marked reduction of myocardial inotropic reserve and continued loss of pump function. Data accumulated over the last two decades indicates that a primary culprit in initiating and maintain βAR dysfunction in the injured and stressed heart is GPCR kinase 2 (GRK2), which was originally known as βARK1 (for βAR kinase). GRK2 is up-regulated in the failing heart due to chronic SNS activity and targeting this kinase has emerged as a novel therapeutic strategy in HF. Indeed, its inhibition or genetic deletion in several disparate animal models of HF including a pre-clinical pig model has shown that GRK2 targeting improves functional and morphological parameters of the failing heart. Moreover, non-βAR properties of GRK2 appear to also contribute to its pathological effects and thus, its inhibition will likely complement existing therapies such as βAR blockade. This review will explore recent research regarding GRK2 inhibition; in particular it will focus on the GRK2 inhibitor peptide known as βARKct, which represents new hope in the treatment against HF progression.
Keywords: G-protein coupled receptors; G-protein-coupled receptor kinase 2; heart failure; β adrenergic system; β blockers.
Figures

References
-
- Aragón J. P., Condit M. E., Bhushan S., Predmore B. L., Patel S. S., Grinsfelder D. B., et al. (2011). Beta 3-adrenoreceptor stimulation ameliorates myocardial ischemia-reperfusion injury via endothelial nitric oxide synthase and neuronal nitric oxide synthase activation. J. Am. Coll. Cardiol. 58, 2683–2691 10.1016/j.jacc.2011.09.033 - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

