Human placental trophoblast invasion and differentiation: a particular focus on Wnt signaling
- PMID: 24133501
- PMCID: PMC3783976
- DOI: 10.3389/fgene.2013.00190
Human placental trophoblast invasion and differentiation: a particular focus on Wnt signaling
Abstract
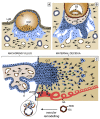
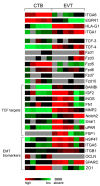
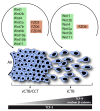
Wingless ligands, a family of secreted proteins, are critically involved in organ development and tissue homeostasis by ensuring balanced rates of stem cell proliferation, cell death and differentiation. Wnt signaling components also play crucial roles in murine placental development controlling trophoblast lineage determination, chorioallantoic fusion and placental branching morphogenesis. However, the role of the pathway in human placentation, trophoblast development and differentiation is only partly understood. Here, we summarize our present knowledge about Wnt signaling in the human placenta and discuss its potential role in physiological and aberrant trophoblast invasion, gestational diseases and choriocarcinoma formation. Differentiation of proliferative first trimester cytotrophoblasts into invasive extravillous trophoblasts is associated with nuclear recruitment of β -catenin and induction of Wnt-dependent T-cell factor 4 suggesting that canonical Wnt signaling could be important for the formation and function of extravillous trophoblasts. Indeed, activation of the pathway was shown to promote trophoblast invasion in different in vitro trophoblast model systems as well as trophoblast cell fusion. Methylation-mediated silencing of inhibitors of Wnt signaling provided evidence for epigenetic activation of the pathway in placental tissues and choriocarcinoma cells. Similarly, abundant nuclear expression of β -catenin in invasive trophoblasts of complete hydatidiform moles suggested a role for hyper-activated Wnt signaling. In contrast, upregulation of Wnt inhibitors was noticed in placentae of women with preeclampsia, a disease characterized by shallow trophoblast invasion and incomplete spiral artery remodeling. Moreover, changes in Wnt signaling have been observed upon cytomegalovirus infection and in recurrent abortions. In summary, the current literature suggests a critical role of Wnt signaling in physiological and abnormal trophoblast function.
Keywords: Wnt; human; invasion; placenta; trophoblast.
Figures

References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

