NME1 suppression of endometrial stromal cells promotes angiogenesis in the endometriotic milieu via stimulating the secretion of IL-8 and VEGF
- PMID: 24133580
- PMCID: PMC3796224
NME1 suppression of endometrial stromal cells promotes angiogenesis in the endometriotic milieu via stimulating the secretion of IL-8 and VEGF
Abstract
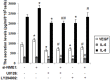
Nonmetastatic gene 23-H1 (NME1, also known as nm23-H1) is a wide-spectrum tumor metastasis suppressor gene that plays an important role in suppressing the proliferation, adhesion and invasion of endometrial stromal cells (ESCs). The present study is undertaken to explore the mechanism by which NME1 in ESCs from endometriosis modulates the angiogenesis and herein participates in the pathogenesis of endometriosis. The expression of NME1 in the primary ESCs from normal endometrium without endometriosis was higher than that from eutopic endometrium and ectopic lesion with endometriosis. Silencing NME1 stimulated the secretion of angiogenic factors interleukin-8 (IL-8) and vascular-endothelial growth factor (VEGF) of the eutopic ESCs from women with endometriosis, and these effects could be abrogated by MAPK/ERK1/2 or AKT inhibitor. In addition, the supernatant of NME1-silenced ESCs increased the expression of angiogenesis-relative molecules CD62E and CD105, and promoted angiogenesis of human umbilical vein endothelial cells (HUVECs). Anti-human IL-8 or VEGF neutralizing antibody reversed the effect on angiogenesis of HUVECs induced by NME1-silenced ESCs. Our current results suggest that the abnormal lower expression of NME1 in ESCs secrete more IL-8 and VEGF through activation of MAPK/ERK1/2 and AKT signal pathways, up-regulate the level of CD62E and CD105, and finally lead to numerous angiogenesis of vascular endothelial cells in the endometriotic milieu, which is beneficial to the origin and development of endometriosis.
Keywords: ESCs; HUVECs; NME1; angiogenesis; endometriosis.
Figures




References
-
- Galle PC. Clinical presentation and diagnosis of endometriosis. Obstet Gynecol Clin North Am. 1989;16:29–42. - PubMed
-
- Groothuis PG, Nap AW, Winterhager E, Grümmer R. Vascular development in endometriosis. Angiogenesis. 2005;8:147–156. - PubMed
-
- Laschke MW, Menger MD. In vitro and in vivo approaches to study angiogenesis in the pathophysiology and therapy of endometriosis. Hum Reprod Update. 2007;13:331–342. - PubMed
-
- May K, Becker CM. Endometriosis and angiogenesis. Minerva Ginecol. 2008;60:245–254. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous
