Preventive effects of low-dose aspirin on colorectal adenoma growth in patients with familial adenomatous polyposis: double-blind, randomized clinical trial
- PMID: 24133627
- PMCID: PMC3797560
- DOI: 10.1002/cam4.46
Preventive effects of low-dose aspirin on colorectal adenoma growth in patients with familial adenomatous polyposis: double-blind, randomized clinical trial
Abstract
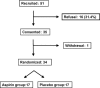
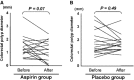
There are several reports of clinical trials of aspirin in sporadic colon cancer. However, only one double-blind trial of aspirin in patients with familial adenomatous polyposis (FAP) has been reported to date. This double-blind, randomized, placebo-controlled clinical trial was therefore performed to evaluate the influence of low-dose aspirin enteric-coated tablets (100 mg/day for 6-10 months) in 34 subjects with FAP (17 each in the aspirin and placebo groups). The increase in mean diameter of colorectal polyps tended to be greater in the placebo group compared with the aspirin group, which showed a response ratio, that is, aspirin response rate (number of subjects with reduced polyps/total)/placebo response rate (number of subjects with reduced polyps/total), of 2.33 (95% confidence interval: 0.72-7.55). Subgroup analysis revealed that the number of subjects with a mean baseline polyp diameter of ≤2 mm, and the diameter and number of polyps after intervention showed a significant reduction in the aspirin group. Adverse effects of aspirin, such as anastomotic ulcer, aphtha in the large intestine, and progression of anemia, occurred in three subjects. Moreover, none of the subjects developed colorectal cancer. The results thus indicated a potential for aspirin to reduce colorectal adenoma development in patients with FAP, but careful follow-up is needed to avoid or rapidly counter severe adverse effects.
Keywords: Adenoma; FAP; chemoprevention; colorectum; low-dose aspirin.
Figures
References
-
- Iwama T., Tamura K., Morita T., Hirai T., Hasegawa H., Koizumi K. Japanese Society for Cancer of the Colon and Rectum. A clinical overview of familial adenomatous polyposis derived from the database of the Polyposis Registry of Japan. Int. J. Clin. Oncol. 2004;9:308–316. - PubMed
-
- Ishikawa H. Chemoprevention of carcinogenesis in familial tumors. Int. J. Clin. Oncol. 2004;9:299–303. - PubMed
-
- Giardiello F. M., Hamilton S. R., Krush A. J., Piantadosi S., Hylind L. M., Celano P. Treatment of colonic and rectal adenomas with sulindac in familial adenomatous polyposis. N. Engl. J. Med. 1993;328:1313–1316. - PubMed
-
- Ishikawa H., Akedo I., Suzuki T., Narahara H., Otani T. Adverse effects of sulindac used for prevention of colorectal cancer. J. Natl. Cancer Inst. 1997;89:1381. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous