Design, synthesis and biological assessment of a triazine dendrimer with approximately 16 Paclitaxel groups and 8 PEG groups
- PMID: 24134039
- PMCID: PMC10262295
- DOI: 10.1021/mp400290u
Design, synthesis and biological assessment of a triazine dendrimer with approximately 16 Paclitaxel groups and 8 PEG groups
Abstract
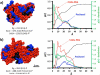
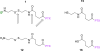
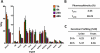
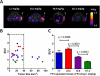
The synthesis and characterization of a generation three triazine dendrimer that displays a phenolic group at the core for labeling, up to eight 5 kDa PEG chains for solubility, and 16 paclitaxel groups is described. Three different diamine linkers--dipiperidine trismethylene, piperazine, and aminomethylpiperidine--were used within the dendrimer. To generate the desired stoichiometric ratio of 8 PEG chains to 16 paclitaxel groups, a monochlorotriazine was prepared with two paclitaxel groups attached through their 2'-hydroxyls using a linker containing a labile disulfide. This monochlorotriazine was linked to a dichlorotriazine with aminomethylpiperidine. The resulting dichlorotriazine bearing two paclitaxel groups could be reacted with the eight amines of the dendrimer. NMR and MALDI-TOF confirm successful reaction. The eight monochlorotriazines of the resulting material are used as the site for PEGylation affording the desired 2:1 stoichiometry. The target and intermediates were amenable to characterization by (1)H and (13)C NMR, and mass spectrometry. Analysis revealed that 16 paclitaxel groups were installed along with 5-8 PEG chains. The final construct is 63% PEG, 22% paclitaxel, and 15% triazine dendrimer. Consistent with previous efforts and computational models, 5 kDa PEG groups were essential for making the target water-soluble. Molecular dynamics simulations showed a high degree of hydration of the core, and a radius of gyration of 2.8 ± 0.2 nm. The hydrodynamic radius of the target was found to be 15.8 nm by dynamic light scattering, an observation indicative of aggregation. Drug release studies performed in plasma showed slow and identical release in mouse and rat plasma (8%, respectively). SPECT/CT imaging was used to follow biodistribution and tumor uptake. Using a two component model, the elimination and distribution half-lives were 2.65 h and 38.2 h, respectively. Compared with previous constructs, this dendrimer persists in the vasculature longer (17.33 ± 0.88% ID/g at 48 h postinjection), and showed higher tumor uptake. Low levels of dendrimer were observed in lung, liver, and spleen (~6% ID/g). Tumor saturation studies of small prostate cancer tumors (PC3) suggest that saturation occurs at a dose between 23.2 mg/kg and 70.9 mg/kg.
Figures
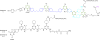


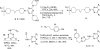
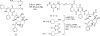
Similar articles
-
Design, synthesis, characterization, and biological evaluation of triazine dendrimers bearing paclitaxel using ester and ester/disulfide linkages.Bioconjug Chem. 2009 Nov;20(11):2154-61. doi: 10.1021/bc900324z. Bioconjug Chem. 2009. PMID: 19877601
-
Biological assessment of triazine dendrimer: toxicological profiles, solution behavior, biodistribution, drug release and efficacy in a PEGylated, paclitaxel construct.Mol Pharm. 2010 Aug 2;7(4):993-1006. doi: 10.1021/mp100104x. Mol Pharm. 2010. PMID: 20481608 Free PMC article.
-
The role of the size and number of polyethylene glycol chains in the biodistribution and tumor localization of triazine dendrimers.Mol Pharm. 2008 Jul-Aug;5(4):540-7. doi: 10.1021/mp8000292. Mol Pharm. 2008. PMID: 18672950
-
Synthesis of water-soluble dendrimers based on melamine bearing 16 paclitaxel groups.Org Lett. 2008 Jan 17;10(2):201-4. doi: 10.1021/ol7024907. Epub 2007 Dec 19. Org Lett. 2008. PMID: 18088131
-
Tumor Uptake of Triazine Dendrimers Decorated with Four, Sixteen, and Sixty-Four PSMA-Targeted Ligands: Passive versus Active Tumor Targeting.Biomolecules. 2019 Aug 28;9(9):421. doi: 10.3390/biom9090421. Biomolecules. 2019. PMID: 31466360 Free PMC article.
Cited by
-
Activatable Dendritic 19F Probes for Enzyme Detection.ACS Macro Lett. 2015 Apr 21;4(4):422-425. doi: 10.1021/acsmacrolett.5b00199. Epub 2015 Apr 1. ACS Macro Lett. 2015. PMID: 25949857 Free PMC article.
-
Nanoparticle Effects on Human Platelets in Vitro: A Comparison between PAMAM and Triazine Dendrimers.Molecules. 2016 Mar 29;21(4):428. doi: 10.3390/molecules21040428. Molecules. 2016. PMID: 27043508 Free PMC article.
-
Nanoparticles as Theranostic Vehicles in Experimental and Clinical Applications-Focus on Prostate and Breast Cancer.Int J Mol Sci. 2017 May 20;18(5):1102. doi: 10.3390/ijms18051102. Int J Mol Sci. 2017. PMID: 28531102 Free PMC article. Review.
-
On-Demand Dissolution of a Dendritic Hydrogel-based Dressing for Second-Degree Burn Wounds through Thiol-Thioester Exchange Reaction.Angew Chem Int Ed Engl. 2016 Aug 16;55(34):9984-7. doi: 10.1002/anie.201604827. Epub 2016 Jul 13. Angew Chem Int Ed Engl. 2016. PMID: 27410669 Free PMC article.
-
Prodrug Strategies for Paclitaxel.Int J Mol Sci. 2016 May 23;17(5):796. doi: 10.3390/ijms17050796. Int J Mol Sci. 2016. PMID: 27223283 Free PMC article. Review.
References
-
- Schellmann N, Deckert PM, Bachran D, Fuchs H, Bachran C. Targeted enzyme prodrug therapies. Mini-Rev. Med. Chem. 2010;10:887–904. - PubMed
-
- Skwarczynski M, Hayashi Y, Kiso Y. Paclitaxel prodrugs: toward smarter delivery of anticancer agents. J. Med. Chem. 2006;49:7253–7269. - PubMed
-
- Chirgwin J, Chua SL. Management of breast cancer with nanoparticle albumin-bound (nab)-paclitaxel combination regimens: a clinical review. Breast. 2011;20:394–406. - PubMed
-
- Croom KF, Dhillon S. Bevacizumab: a review of its use in combination with paclitaxel or capecitabine as first-line therapy for HER2-negative metastatic breast cancer. Drugs. 2011;71:2213–2229. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

